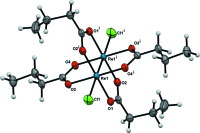
Crystal structure of tetra-kis-(μ-n-butyrato-κ(2) O:O')bis-[chlorido-rhenium(III)](Re-Re)
- PMID: 25484675
- PMCID: PMC4257215
- DOI: 10.1107/S1600536814020273
Crystal structure of tetra-kis-(μ-n-butyrato-κ(2) O:O')bis-[chlorido-rhenium(III)](Re-Re)
Abstract
With an inversion center at the mid-point of the two Re(III) atoms, the title compound, [Re2Cl2{O2C(CH2)2CH3}4], exhibits a paddle-wheel or lantern-type structure with four n-butyrate groups bridging two Re(III) atoms in a syn-syn fashion. The axial chloride ligands together with the Re-Re quadruple bond [2.2330 (3) Å] complete an essentially octa-hedral geometry around each Re(III) atom. There is little distortion, with an Re-Re-Cl bond angle of 176.18 (3)° and typical cis-O-Re-O bond angles ranging from 89.39 (11) to 90.68 (11)°. There are two mol-ecules in the unit cell, and no significant inter-molecular inter-actions were noticed between mol-ecules in the crystal.
Keywords: butyrate bridging ligand; crystal structure; dirhenium core.
Figures
References
-
- Bruker (2010). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Calvo, C., Jayadevan, N. C. & Lock, C. J. L. (1969). Can. J. Chem. 47, 4213–4220.
-
- Collins, D. M., Cotton, F. A. & Gage, L. D. (1979). Inorg. Chem. 18, 1712–1715.
-
- Lydon, D. P., Spalding, T. R. & Gallagher, J. F. (2003). Polyhedron, 22, 1281–1287.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous