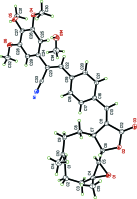
Crystal structure of (E)-13-{4-[(Z)-2-cyano-2-(3,4,5-tri-meth-oxy-phen-yl)ethen-yl]phen-yl}parthenolide methanol hemisolvate
- PMID: 25484689
- PMCID: PMC4257209
- DOI: 10.1107/S1600536814019333
Crystal structure of (E)-13-{4-[(Z)-2-cyano-2-(3,4,5-tri-meth-oxy-phen-yl)ethen-yl]phen-yl}parthenolide methanol hemisolvate
Abstract
The title compound, C33H35NO6 [systematic name: (Z)-3-(4-{(E)-[(E)-1a,5-dimethyl-9-oxo-2,3,7,7a-tetra-hydro-oxireno[2',3':9,10]cyclo-deca-[1,2-b]furan-8(1aH,6H,9H,10aH,10bH)-yl-idene]meth-yl}phen-yl)-2-(3,4,5-tri-meth-oxy-phen-yl)acrylo-ni-trile methanol hemisolvate], C33H35NO6·0.5CH3OH, was prepared by the reaction of (Z)-3-(4-iodo-phen-yl)-2-(3,4,5-tri-meth-oxy-phen-yl)acrylo-nitrile with parthenolide [systematic name: (E)-1a,5-dimethyl-8-methyl-ene-2,3,6,7,7a,8,10a,10b-octa-hy-dro-oxireno[2',3':9,10]cyclo-deca-[1,2-b]furan-9(1aH)-one] under Heck reaction conditions. The mol-ecule is built up from fused ten-, five- (lactone) and three-membered (epoxide) rings with a {4-[(Z)-2-cyano-2-(3,4,5-tri-meth-oxy-phen-yl)ethen-yl]phen-yl}methyl-idene group as a substituent. The 4-[(Z)-2-cyano-2-(3,4,5-tri-meth-oxy-phen-yl)ethen-yl]phenyl group on the parthenolide exocyclic double bond is oriented in a trans position to the lactone ring to form the E isomer. The dihedral angle between the benzene ring of the phenyl moiety and the lactone ring mean plane is 21.93 (4)°.
Keywords: Heck synthesis; biological activity; crystal structure; parthenolide derivatives.
Figures
References
-
- Bruker (2006). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Hall, I. H., Lee, K. H., Starnes, C. O., Sumida, Y., Wu, R. Y., Waddell, T. G., Cochran, J. W. & Gerhart, K. G. (1979). J. Pharm. Sci. 68, 537–542. - PubMed
-
- Han, C., Barrios, F. J., Riofski, M. V. & Colby, D. A. (2009). J. Org. Chem. 74, 7176–7179. - PubMed
-
- Hanson, R. L., Lardy, H. A. & Kupchan, S. M. (1970). Science, 168, 378–380. - PubMed
-
- Hehner, S. P., Heinrich, M., Bork, P. M., Vogt, M., Ratter, F., Lehmann, V., Osthoff, K. S., Dröge, W. & Schmitz, M. L. (1998). J. Biol. Chem. 273, 1288–1297. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources