Crystal structure of flufenoxuron: a benzoyl-urea pesticide
- PMID: 25484700
- PMCID: PMC4257195
- DOI: 10.1107/S1600536814020649
Crystal structure of flufenoxuron: a benzoyl-urea pesticide
Abstract
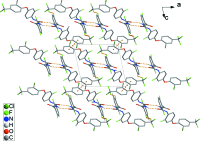
The title compound, C21H11ClF6N2O3 (systematic name: 1-{4-[2-chloro-4-(trifluoromethyl)phenoxy]-2-fluorophenyl}-3-(2,6-di-fluoro-benzo-yl)urea), is a benzoyl-urea pesticide. The dihedral angles between the central fluoro-benzene ring and the terminal di-fluoro-phenyl ring and chloro-phenyl ring system are 62.15 (5) and 88.03 (5)°, respectively. In the crystal, N-H⋯O hydrogen bonds link adjacent mol-ecules, forming R 2 (2)(8) inversion dimers that pack into loop chains along the a-axis direction by short F⋯F contacts [2.729 (2) Å]. In addition, the chains are linked by weak C-H⋯π and π-π inter-actions [inter-centroid distances = 3.661 (2) and 3.535 (12) Å], resulting in a three-dimensional architecture.
Keywords: C—H⋯π interactions; N—H⋯O hydrogen bonds; benzoylurea; crystal structure; pesticide; π–π interactions.
Figures

References
-
- Brandenburg, K. (2010). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Kamel, A., Al-Dosary, S., Ibrahim, S. & Ahmed, M. A. (2007). Food Chem. 100, 1590–1593.
-
- Salokhe, S., Sarkar, A., Kulkarni, A., Mukherjee, S. & Pal, J. K. (2006). Pestic. Biochem. Physiol. 85, 84–90.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
