Crystal structures of (R S)-N-[(1R,2S)-2-benz-yloxy-1-(2,6-di-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide and (R S)-N-[(1S,2R)-2-benz-yloxy-1-(2,4,6-tri-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide: two related protected 1,2-amino alcohols
- PMID: 25484747
- PMCID: PMC4257314
- DOI: 10.1107/S1600536814022570
Crystal structures of (R S)-N-[(1R,2S)-2-benz-yloxy-1-(2,6-di-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide and (R S)-N-[(1S,2R)-2-benz-yloxy-1-(2,4,6-tri-methyl-phen-yl)prop-yl]-2-methyl-propane-2-sulfinamide: two related protected 1,2-amino alcohols
Abstract
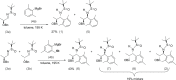
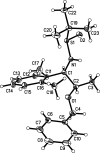
The title compounds, C22H31NO2S, (1), and C23H33NO2S, (2), are related protected 1,2-amino alcohols. They differ in the substituents on the benzene ring, viz. 2,6-di-methyl-phenyl in (1) and 2,4,6-tri-methyl-phenyl in (2). The plane of the phenyl ring is inclined to that of the benzene ring by 28.52 (7)° in (1) and by 44.65 (19)° in (2). In the crystal of (1), N-H⋯O=S and C-H⋯O=S hydrogen bonds link mol-ecules, forming chains along [100], while in (2), similar hydrogen bonds link mol-ecules into chains along [010]. The absolute structures of both compounds were determined by resonance scattering.
Keywords: NMR; amino alcohol; column chromatography; crystal structure; diastereomer; hydrogen bonding; sulfinamide.
Figures



References
-
- Ager, D. J., Prakash, I. & Schaad, D. R. (1996). Chem. Rev. 96, 835–876. - PubMed
-
- Bruker (2014). APEX2, SAINT and SADABS. Bruker AXS, Inc., Madison, Wisconsin, USA.
-
- Buesking, A. W. & Ellman, J. A. (2014). Chem. Sci. 5, 1983–1987.
-
- Enders, D., von Berg, S., Jandeleit, B., Savall, B. M. & Roush, W. R. (2002). Org. Synth. 78, 177–182.
-
- Evans, J. W. & Ellman, J. A. (2003). J. Org. Chem. 68, 9948–9957. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
