Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-α expression in 7 month old unchallenged mice
- PMID: 25484856
- PMCID: PMC4240169
- DOI: 10.3389/fncel.2014.00399
Long-term omega-3 supplementation modulates behavior, hippocampal fatty acid concentration, neuronal progenitor proliferation and central TNF-α expression in 7 month old unchallenged mice
Abstract
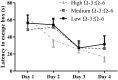
Dietary polyunsaturated fatty acid (PUFA) manipulation is being investigated as a potential therapeutic supplement to reduce the risk of developing age-related cognitive decline (ARCD). Animal studies suggest that high omega (Ω)-3 and low Ω-6 dietary content reduces cognitive decline by decreasing central nervous system (CNS) inflammation and modifying neuroimmune activity. However, no previous studies have investigated the long term effects of Ω-3 and Ω-6 dietary levels in healthy aging mice leaving the important question about the preventive effects of Ω-3 and Ω-6 on behavior and underlying molecular pathways unaddressed. We aimed to investigate the efficacy of long-term Ω-3 and Ω-6 PUFA dietary supplementation in mature adult C57BL/6 mice. We measured the effect of low, medium, and high Ω-3:Ω-6 dietary ratio, given from the age of 3-7 months, on anxiety and cognition-like behavior, hippocampal tissue expression of TNF-α, markers of neuronal progenitor proliferation and gliogenesis and serum cytokine concentration. Our results show that a higher Ω-3:Ω-6 PUFA diet ratio increased hippocampal PUFA, increased anxiety, improved hippocampal dependent spatial memory and reduced hippocampal TNF-α levels compared to a low Ω-3:Ω-6 diet. Furthermore, serum TNF-α concentration was reduced in the higher Ω-3:Ω-6 PUFA ratio supplementation group while expression of the neuronal progenitor proliferation markers KI67 and doublecortin (DCX) was increased in the dentate gyrus as opposed to the low Ω-3:Ω-6 group. Conversely, Ω-3:Ω-6 dietary PUFA ratio had no significant effect on astrocyte or microglia number or cell death in the dentate gyrus. These results suggest that supplementation of PUFAs may delay aging effects on cognitive function in unchallenged mature adult C57BL/6 mice. This effect is possibly induced by increasing neuronal progenitor proliferation and reducing TNF-α.
Keywords: TNF-α; cognition; neurogenesis; omega 3; polyunsaturated fatty acid.
Figures







Similar articles
-
Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia.Int J Mol Sci. 2022 Mar 21;23(6):3399. doi: 10.3390/ijms23063399. Int J Mol Sci. 2022. PMID: 35328825 Free PMC article.
-
Impact of dietary n-3 polyunsaturated fatty acids on cognition, motor skills and hippocampal neurogenesis in developing C57BL/6J mice.J Nutr Biochem. 2015 Jan;26(1):24-35. doi: 10.1016/j.jnutbio.2014.08.002. Epub 2014 Sep 28. J Nutr Biochem. 2015. PMID: 25444517
-
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article.
-
Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: Efficacy declines with aging.Neurobiol Dis. 2019 Jun;126:62-75. doi: 10.1016/j.nbd.2018.09.012. Epub 2018 Sep 12. Neurobiol Dis. 2019. PMID: 30218758 Review.
-
Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms.Ageing Res Rev. 2013 Mar;12(2):579-94. doi: 10.1016/j.arr.2013.01.007. Epub 2013 Feb 6. Ageing Res Rev. 2013. PMID: 23395782 Review.
Cited by
-
N-3 Polyunsaturated Fatty Acids through the Lifespan: Implication for Psychopathology.Int J Neuropsychopharmacol. 2016 Dec 30;19(12):pyw078. doi: 10.1093/ijnp/pyw078. Print 2016 Dec. Int J Neuropsychopharmacol. 2016. PMID: 27608809 Free PMC article. Review.
-
Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia.Int J Mol Sci. 2022 Mar 21;23(6):3399. doi: 10.3390/ijms23063399. Int J Mol Sci. 2022. PMID: 35328825 Free PMC article.
-
Protective effects of omega-3 against procarbazine-induced brain damage in the cerebellum and CA1 Hippocampus of male rats: a focus on oxidative stress mechanisms.Metab Brain Dis. 2025 Mar 15;40(3):153. doi: 10.1007/s11011-025-01575-0. Metab Brain Dis. 2025. PMID: 40088343
-
Behavioral, neuromorphological, and neurobiochemical effects induced by omega-3 fatty acids following basal forebrain cholinergic depletion in aged mice.Alzheimers Res Ther. 2020 Nov 16;12(1):150. doi: 10.1186/s13195-020-00705-3. Alzheimers Res Ther. 2020. PMID: 33198763 Free PMC article.
-
Non-Alcoholic Fatty Liver Disease, and the Underlying Altered Fatty Acid Metabolism, Reveals Brain Hypoperfusion and Contributes to the Cognitive Decline in APP/PS1 Mice.Metabolites. 2019 May 25;9(5):104. doi: 10.3390/metabo9050104. Metabolites. 2019. PMID: 31130652 Free PMC article.
References
-
- Belzung C., Leguisquet A. M., Barreau S., Delion-Vancassel S., Chalon S., Durand G. (1998). Alpha-linolenic acid deficiency modifies distractibility but not anxiety and locomotion in rats during aging. J. Nutr. 128, 1537–1542. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

