ITI-Signals and Prelimbic Cortex Facilitate Avoidance Acquisition and Reduce Avoidance Latencies, Respectively, in Male WKY Rats
- PMID: 25484860
- PMCID: PMC4240176
- DOI: 10.3389/fnbeh.2014.00403
ITI-Signals and Prelimbic Cortex Facilitate Avoidance Acquisition and Reduce Avoidance Latencies, Respectively, in Male WKY Rats
Abstract
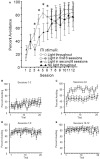
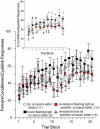
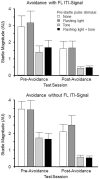
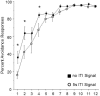
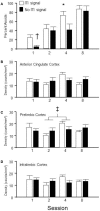
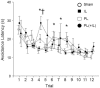
As a model of anxiety disorder vulnerability, male Wistar-Kyoto (WKY) rats acquire lever-press avoidance behavior more readily than outbred Sprague-Dawley rats, and their acquisition is enhanced by the presence of a discrete signal presented during the inter-trial intervals (ITIs), suggesting that it is perceived as a safety signal. A series of experiments were conducted to determine if this is the case. Additional experiments investigated if the avoidance facilitation relies upon processing through medial prefrontal cortex (mPFC). The results suggest that the ITI-signal facilitates acquisition during the early stages of the avoidance acquisition process, when the rats are initially acquiring escape behavior and then transitioning to avoidance behavior. Post-avoidance introduction of the visual ITI-signal into other associative learning tasks failed to confirm that the visual stimulus had acquired the properties of a conditioned inhibitor. Shortening the signal from the entirety of the 3 min ITI to only the first 5 s of the 3 min ITI slowed acquisition during the first four sessions, suggesting the flashing light (FL) is not functioning as a feedback signal. The prelimbic (PL) cortex showed greater activation during the period of training when the transition from escape responding to avoidance responding occurs. Only combined PL + infralimbic cortex lesions modestly slowed avoidance acquisition, but PL-cortex lesions slowed avoidance response latencies. Thus, the FL ITI-signal is not likely perceived as a safety signal nor is it serving as a feedback signal. The functional role of the PL-cortex appears to be to increase the drive toward responding to the threat of the warning signal. Hence, avoidance susceptibility displayed by male WKY rats may be driven, in part, both by external stimuli (ITI signal) as well as by enhanced threat recognition to the warning signal via the PL cortex.
Keywords: anxiety vulnerability; conditioned inhibitor; infralimbic cortex; lever-press avoidance; prelimbic cortex; safety signals.
Figures
Similar articles
-
Partial Predictability in Avoidance Acquisition and Expression of Wistar-Kyoto and Sprague-Dawley Rats: Implications for Anxiety Vulnerability in Uncertain Situations.Front Psychiatry. 2020 Aug 19;11:848. doi: 10.3389/fpsyt.2020.00848. eCollection 2020. Front Psychiatry. 2020. PMID: 32973587 Free PMC article.
-
Vulnerability factors in anxiety: Strain and sex differences in the use of signals associated with non-threat during the acquisition and extinction of active-avoidance behavior.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Aug 15;35(7):1659-70. doi: 10.1016/j.pnpbp.2011.05.002. Epub 2011 May 13. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21601608
-
Paired-housing selectively facilitates within-session extinction of avoidance behavior, and increases c-Fos expression in the medial prefrontal cortex, in anxiety vulnerable Wistar-Kyoto rats.Physiol Behav. 2016 Oct 1;164(Pt A):198-206. doi: 10.1016/j.physbeh.2016.05.044. Epub 2016 May 24. Physiol Behav. 2016. PMID: 27235339
-
Rapid avoidance acquisition in Wistar-Kyoto rats.Behav Brain Res. 2008 Oct 10;192(2):191-7. doi: 10.1016/j.bbr.2008.04.006. Epub 2008 Apr 18. Behav Brain Res. 2008. PMID: 18501974
-
Activation of extracellular signal-regulated kinase (ERK) and ΔFosB in emotion-associated neural circuitry after asymptotic levels of active avoidance behavior are attained.Brain Res Bull. 2013 Sep;98:102-10. doi: 10.1016/j.brainresbull.2013.07.004. Epub 2013 Aug 6. Brain Res Bull. 2013. PMID: 23932962
Cited by
-
Avoidance expression in rats as a function of signal-shock interval: strain and sex differences.Front Behav Neurosci. 2015 Jul 6;9:168. doi: 10.3389/fnbeh.2015.00168. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26217200 Free PMC article.
-
Divergent encoding of active avoidance behavior in corticostriatal and corticolimbic projections.Sci Rep. 2022 Jun 24;12(1):10731. doi: 10.1038/s41598-022-14930-3. Sci Rep. 2022. PMID: 35750718 Free PMC article.
-
Partial Predictability in Avoidance Acquisition and Expression of Wistar-Kyoto and Sprague-Dawley Rats: Implications for Anxiety Vulnerability in Uncertain Situations.Front Psychiatry. 2020 Aug 19;11:848. doi: 10.3389/fpsyt.2020.00848. eCollection 2020. Front Psychiatry. 2020. PMID: 32973587 Free PMC article.
-
Behavioral outputs and overlapping circuits between conditional fear and active avoidance.Neurobiol Learn Mem. 2024 Sep;213:107943. doi: 10.1016/j.nlm.2024.107943. Epub 2024 May 29. Neurobiol Learn Mem. 2024. PMID: 38821256 Free PMC article. Review.
-
Active avoidance requires inhibitory signaling in the rodent prelimbic prefrontal cortex.Elife. 2018 May 31;7:e34657. doi: 10.7554/eLife.34657. Elife. 2018. PMID: 29851381 Free PMC article.
References
-
- Beck K. D., Jiao X., Ricart T. M., Myers C. E., Minor T. R., Pang K. C., et al. (2011). Vulnerability factors in anxiety: strain and sex differences in the use of signals associated with non-threat during the acquisition and extinction of active-avoidance behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1659–1670.10.1016/j.pnpbp.2011.05.002 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

