A novel mouse model of creatine transporter deficiency
- PMID: 25485098
- PMCID: PMC4243761
- DOI: 10.12688/f1000research.5369.1
A novel mouse model of creatine transporter deficiency
Abstract
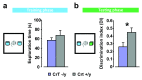
Mutations in the creatine (Cr) transporter (CrT) gene lead to cerebral creatine deficiency syndrome-1 (CCDS1), an X-linked metabolic disorder characterized by cerebral Cr deficiency causing intellectual disability, seizures, movement and behavioral disturbances, language and speech impairment ( OMIM #300352). CCDS1 is still an untreatable pathology that can be very invalidating for patients and caregivers. Only two murine models of CCDS1, one of which is an ubiquitous knockout mouse, are currently available to study the possible mechanisms underlying the pathologic phenotype of CCDS1 and to develop therapeutic strategies. Given the importance of validating phenotypes and efficacy of promising treatments in more than one mouse model we have generated a new murine model of CCDS1 obtained by ubiquitous deletion of 5-7 exons in the Slc6a8 gene. We showed a remarkable Cr depletion in the murine brain tissues and cognitive defects, thus resembling the key features of human CCDS1. These results confirm that CCDS1 can be well modeled in mice. This CrT (-/y) murine model will provide a new tool for increasing the relevance of preclinical studies to the human disease.
Conflict of interest statement
Figures






References
-
- Baroncelli L, Alessandrì MG, Tola J, et al. : Dataset 1. Data for neurochemical and behavioral assessment in a mouse model of creatine deficiency. F1000Research. 2014. Data Source - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

