Indirect shoot organogenesis from leaf explants of Adhatoda vasica Nees
- PMID: 25485191
- PMCID: PMC4230685
- DOI: 10.1186/2193-1801-3-648
Indirect shoot organogenesis from leaf explants of Adhatoda vasica Nees
Abstract
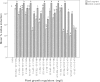
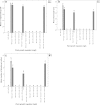
A novel protocol for indirect shoot organogenesis of Adhatoda vasica was developed using petiole explants derived from mature shrubby plants. Media with concentrations of cytokinins in combination with auxins were used to induce callus formation in two explants types: petiole and leaf segment. The frequency of callus formation from petiole and leaf segment explants on Murashige and Skoog (MS) basal medium supplemented with 0.25 mg l(-1) thidiazuron (TDZ) and 0.25 mg l(-1) α-naphthaleneacetic acid (NAA) was 100 ± 0.0 and 83.70 ± 0.52% respectively, while on this medium supplemented with 0.25 mg l(-1) 6-(γ-γ, dimethylallyamino purine) (2iP) and 0.25 mg l(-1) NAA, the callus frequency was 100 ± 0.0 and 96.70 ± 0.67% respectively. The highest shoot regeneration (90.60 ± 0.52%) response and the maximum shoots (8.10 ± 0.28) per callus were achieved from petiole explants on MS medium containing 0.25 mg l(-1) TDZ and 0.25 mg l(-1) NAA. On the contrary, on Schenk & Hildebrandt (SH) basal medium supplemented with 0.25 mg l(-1) TDZ and 0.25 mg l(-1) NAA, the frequency of callus formation from petiole and leaf segment explants was 100 ± 0.0 and 90.50 ± 0.89% respectively while the callus frequency on this medium containing 0.25 mg l(-1) 2iP and 0.25 mg l(-1) NAA was 100 ± 0.0 and 89.90 ± 0.72% respectively. The shoot regeneration frequency for petiole explants was 89.90 ± 0.46% producing 6.00 ± 0.21 shoots per callus on SH basal medium supplemented with 0.25 mg l(-1) TDZ and 0.25 mg l(-1) NAA. Whereas petiole explants could induce 83.70 ± 0.50% shoot regeneration and 7.3 ± 1.05 shoots per callus on SH medium containing 0.25 mg l(-1) indole-3-butyric acid (IBA), 0.5 mg l(-1) 6-benzyladenine (BA) and 0.5 mg l(-1) 2iP. Elongation of regenerated shoot was obtained on MS basal medium supplemented with 0.25 mg l(-1) TDZ. All regenerated shoots developed adventitious roots within 4 weeks when transferred to rooting medium containing SH medium supplemented with 0.5 mg l(-1) IBA. Total nine rooted plantlets were transferred from in vitro to in vivo conditions and eight plants survived and successfully acclimatized in the shaded greenhouse 12 weeks after transplanting.
Keywords: Adhatoda vasica; Adventitious shoots; Callus induction; Organogenesis; Shoot regeneration.
Figures


Similar articles
-
In vitro biotechnological advancements in Malabar nut (Adhatoda vasica Nees): Achievements, status and prospects.J Genet Eng Biotechnol. 2018 Dec;16(2):545-552. doi: 10.1016/j.jgeb.2018.03.007. Epub 2018 Mar 19. J Genet Eng Biotechnol. 2018. PMID: 30733772 Free PMC article. Review.
-
High-frequency adventitious shoot organogenesis from in vitro stem explants of Scutellaria araxensis Grossh.BioTechnologia (Pozn). 2022 Jun 29;103(2):143-151. doi: 10.5114/bta.2022.116208. eCollection 2022. BioTechnologia (Pozn). 2022. PMID: 36606069 Free PMC article.
-
Induction, Subculture Cycle, and Regeneration of Callus in Safed Musli (Chlorophytum borivilianum) using Different Types of Phytohormones.Pharmacogn Mag. 2016 Jul;12(Suppl 4):S460-S464. doi: 10.4103/0973-1296.191457. Pharmacogn Mag. 2016. PMID: 27761075 Free PMC article.
-
High frequency regeneration of plants via callus-mediated organogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia Cadamba).Sci Rep. 2020 Mar 12;10(1):4558. doi: 10.1038/s41598-020-61612-z. Sci Rep. 2020. PMID: 32165694 Free PMC article.
-
Microbulb and plantlet formation of a native bulbous flower, Lilium monodelphum M. Bieb, var. Armenum, through tissue culture propagation.Biotechnol Rep (Amst). 2021 Aug 8;32:e00665. doi: 10.1016/j.btre.2021.e00665. eCollection 2021 Dec. Biotechnol Rep (Amst). 2021. PMID: 34540598 Free PMC article. Review.
Cited by
-
In vitro biotechnological advancements in Malabar nut (Adhatoda vasica Nees): Achievements, status and prospects.J Genet Eng Biotechnol. 2018 Dec;16(2):545-552. doi: 10.1016/j.jgeb.2018.03.007. Epub 2018 Mar 19. J Genet Eng Biotechnol. 2018. PMID: 30733772 Free PMC article. Review.
References
-
- Abhyankar G, Reddy VD. Rapid micropropagation via axillary bud propliferation of Adhatoda vasica Nees from nodal segments. Indian J Exp Biol. 2007;45:268–271. - PubMed
-
- Amin MN, Azad MAK, Begum F. In vitro plant regeneration from leaf derived callus cultures of Adhatoda vasica Nees. Plant Tissue Cult. 1997;7(2):109–115.
-
- Anand Y, Bansal YK. Synthetic seeds: a novel approach of in vitro plant formation in vasaka (Adhatoda vasica Nees.) Plant Biotechnol. 2002;19(3):159–162. doi: 10.5511/plantbiotechnology.19.159. - DOI
-
- Arshad M, Silvestre J, Merlina G, Dumat C, Pinelli E, Kallerhoff J. Thidiazuron-induced shoot organogenesis from mature leaf explants of scented Pelargonium capitatum cultivars. Plant Cell Tissue Organ Cult. 2012;108:315–322. doi: 10.1007/s11240-011-0045-1. - DOI
-
- Azad MAK, Amin MN. In vitro regeneration of plantlets from internode explants of Adhatoda vasica Nees. Plant Tissue Cult. 1998;8:27.
LinkOut - more resources
Full Text Sources
Other Literature Sources

