LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis
- PMID: 25485679
- PMCID: PMC4382240
- DOI: 10.1172/JCI76357
LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis
Abstract
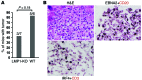
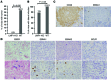
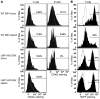
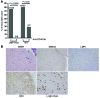
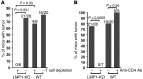
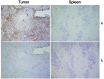
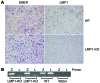
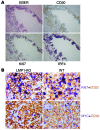
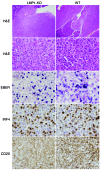
Epstein-Barr virus (EBV) infection transforms B cells in vitro and is associated with human B cell lymphomas. The major EBV oncoprotein, latent membrane protein 1 (LMP1), mimics constitutively active CD40 and is essential for outgrowth of EBV-transformed B cells in vitro; however, EBV-positive diffuse large B cell lymphomas and Burkitt lymphomas often express little or no LMP1. Thus, EBV may contribute to the development and maintenance of human lymphomas even in the absence of LMP1. Here, we found that i.p. injection of human cord blood mononuclear cells infected with a LMP1-deficient EBV into immunodeficient mice induces B cell lymphomas. In this model, lymphoma development required the presence of CD4+ T cells in cord blood and was inhibited by CD40-blocking Abs. In contrast, LMP1-deficient EBV established persistent latency but did not induce lymphomas when directly injected into mice engrafted with human fetal CD34+ cells and human thymus. WT EBV induced lymphomas in both mouse models and did not require coinjected T cells in the cord blood model. Together, these results demonstrate that LMP1 is not essential for EBV-induced lymphomas in vivo and suggest that T cells supply signals that substitute for LMP1 in EBV-positive B cell lymphomagenesis.
Figures


References
-
- Vereide D, Sugden B. Insights into the evolution of lymphomas induced by Epstein-Barr virus. Adv Cancer Res. 2010;108:1–19. - PubMed
-
- Kieff ED, Rickinson AB. Epstein-Barr Virus and its replication. In: Fields BN, Knipe DM, Howley PM, Griffin DE eds. Fields’ Virology. 5th ed. Philadelphia, Pennsylvania, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007:2603–2654.
-
- Rickinson AB, Kieff ED. Epstein-Barr Virus. In: Fields BN, Knipe DM, Howley PM, Griffin DE eds. Fields’ Virology. 5th ed. Philadelphia, Pennsylvania, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007:2655–2700.
-
- Rastelli J, et al. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood. 2008;111(3):1448–1455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

