Increased N-glycosylation efficiency by generation of an aromatic sequon on N135 of antithrombin
- PMID: 25485983
- PMCID: PMC4259341
- DOI: 10.1371/journal.pone.0114454
Increased N-glycosylation efficiency by generation of an aromatic sequon on N135 of antithrombin
Erratum in
-
Correction: Increased N-glycosylation efficiency by generation of an aromatic sequon on N135 of antithrombin.PLoS One. 2015 Mar 23;10(3):e0122177. doi: 10.1371/journal.pone.0122177. eCollection 2015. PLoS One. 2015. PMID: 25799579 Free PMC article. No abstract available.
Abstract
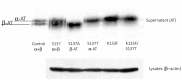
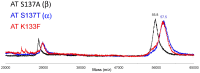
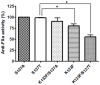
The inefficient glycosylation of consensus sequence on N135 in antithrombin explains the two glycoforms of this key anticoagulant serpin found in plasma: α and β, with four and three N-glycans, respectively. The lack of this N-glycan increases the heparin affinity of the β-glycoform. Recent studies have demonstrated that an aromatic sequon (Phe-Y-Asn-X-Thr) in reverse β-turns enhances N-glycosylation efficiency and stability of different proteins. We evaluated the effect of the aromatic sequon in this defective glycosylation site of antithrombin, despite of being located in a loop between the helix D and the strand 2A. We analyzed the biochemical and functional features of variants generated in a recombinant cell system (HEK-EBNA). Cells transfected with wild-type plasmid (K133-Y-N135-X-S137) generated 50% of α and β-antithrombin. The S137T, as previously reported, K133F, and the double mutant (K133F/S137T) had improved glycosylation efficiency, leading to the secretion of α-antithrombin, as shown by electrophoretic and mass analysis. The presence of the aromatic sequon did not significantly affect the stability of this conformationally sensitive serpin, as revealed by thermal denaturation assay. Moreover, the aromatic sequon hindered the activation induced by heparin, in which is involved the helix D. Accordingly, K133F and particularly K133F/S137T mutants had a reduced anticoagulant activity. Our data support that aromatic sequons in a different structural context from reverse turns might also improve the efficiency of N-glycosylation.
Conflict of interest statement
Figures
Similar articles
-
Effect of individual carbohydrate chains of recombinant antithrombin on heparin affinity and on the generation of glycoforms differing in heparin affinity.Arch Biochem Biophys. 1997 May 15;341(2):212-21. doi: 10.1006/abbi.1997.9973. Arch Biochem Biophys. 1997. PMID: 9169007
-
Partial glycosylation of antithrombin III asparagine-135 is caused by the serine in the third position of its N-glycosylation consensus sequence and is responsible for production of the beta-antithrombin III isoform with enhanced heparin affinity.Biochemistry. 1995 Jul 4;34(26):8433-40. doi: 10.1021/bi00026a026. Biochemistry. 1995. PMID: 7599134
-
Glycosylation of the enhanced aromatic sequon is similarly stabilizing in three distinct reverse turn contexts.Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14127-32. doi: 10.1073/pnas.1105880108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825145 Free PMC article.
-
Effects of glycosylation on heparin binding and antithrombin activation by heparin.Proteins. 2011 Sep;79(9):2735-45. doi: 10.1002/prot.23102. Epub 2011 Jul 18. Proteins. 2011. PMID: 21769943
-
The oligosaccharide side chain on Asn-135 of alpha-antithrombin, absent in beta-antithrombin, decreases the heparin affinity of the inhibitor by affecting the heparin-induced conformational change.Biochemistry. 1997 Jun 3;36(22):6682-91. doi: 10.1021/bi9702492. Biochemistry. 1997. PMID: 9184148
Cited by
-
Correction: Increased N-glycosylation efficiency by generation of an aromatic sequon on N135 of antithrombin.PLoS One. 2015 Mar 23;10(3):e0122177. doi: 10.1371/journal.pone.0122177. eCollection 2015. PLoS One. 2015. PMID: 25799579 Free PMC article. No abstract available.
-
Mechanism of antithrombin deficiency due to the novel variant C32W in the C-terminus of the signal peptide.Int J Hematol. 2025 Jul;122(1):35-44. doi: 10.1007/s12185-025-03945-x. Epub 2025 Feb 10. Int J Hematol. 2025. PMID: 39928217
-
Clinical and Molecular Characterization of Nine Novel Antithrombin Mutations.Int J Mol Sci. 2024 Mar 1;25(5):2893. doi: 10.3390/ijms25052893. Int J Mol Sci. 2024. PMID: 38474138 Free PMC article.
-
Two SERPINC1 variants affecting N-glycosylation of Asn224 cause severe thrombophilia not detected by functional assays.Blood. 2022 Jul 14;140(2):140-151. doi: 10.1182/blood.2021014708. Blood. 2022. PMID: 35486842 Free PMC article.
-
N-Glycosylation as a Tool to Study Antithrombin Secretion, Conformation, and Function.Int J Mol Sci. 2021 Jan 6;22(2):516. doi: 10.3390/ijms22020516. Int J Mol Sci. 2021. PMID: 33419227 Free PMC article.
References
-
- Schwarz F, Aebi M (2011) Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol 21:576–582. - PubMed
-
- Schenk B, Fernandez F, Waechter CJ (2001) The ins(ide) and out(side) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology 11:61R–70R. - PubMed
-
- Preston RJ, Rawley O, Gleeson EM, O′Donnell JS (2013) Elucidating the role of carbohydrate determinants in regulating hemostasis: insights and opportunities. Blood 121:3801–3810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

