A Fox2-dependent fatty acid ß-oxidation pathway coexists both in peroxisomes and mitochondria of the ascomycete yeast Candida lusitaniae
- PMID: 25486052
- PMCID: PMC4259357
- DOI: 10.1371/journal.pone.0114531
A Fox2-dependent fatty acid ß-oxidation pathway coexists both in peroxisomes and mitochondria of the ascomycete yeast Candida lusitaniae
Abstract
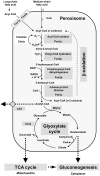
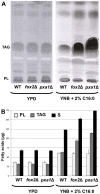
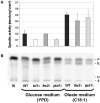
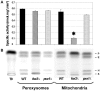
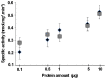
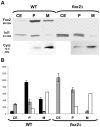
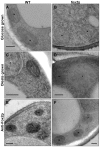
It is generally admitted that the ascomycete yeasts of the subphylum Saccharomycotina possess a single fatty acid ß-oxidation pathway located exclusively in peroxisomes, and that they lost mitochondrial ß-oxidation early during evolution. In this work, we showed that mutants of the opportunistic pathogenic yeast Candida lusitaniae which lack the multifunctional enzyme Fox2p, a key enzyme of the ß-oxidation pathway, were still able to grow on fatty acids as the sole carbon source, suggesting that C. lusitaniae harbored an alternative pathway for fatty acid catabolism. By assaying 14Cα-palmitoyl-CoA consumption, we demonstrated that fatty acid catabolism takes place in both peroxisomal and mitochondrial subcellular fractions. We then observed that a fox2Δ null mutant was unable to catabolize fatty acids in the mitochondrial fraction, thus indicating that the mitochondrial pathway was Fox2p-dependent. This finding was confirmed by the immunodetection of Fox2p in protein extracts obtained from purified peroxisomal and mitochondrial fractions. Finally, immunoelectron microscopy provided evidence that Fox2p was localized in both peroxisomes and mitochondria. This work constitutes the first demonstration of the existence of a Fox2p-dependent mitochondrial β-oxidation pathway in an ascomycetous yeast, C. lusitaniae. It also points to the existence of an alternative fatty acid catabolism pathway, probably located in peroxisomes, and functioning in a Fox2p-independent manner.
Conflict of interest statement
Figures
References
-
- Brock M (2009) Fungal metabolism in host niches. Curr Opin Microbiol 12:371–376. - PubMed
-
- Hiltunen JK, Mursula AM, Rottensteiner H, Wierenga RK, Kastaniotis AJ, et al. (2003) The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae . FEMS Microbiol Rev 27:35–64. - PubMed
-
- Moreno de la Garza M, Schultz-Borchard U, Crabb JW, Kunau W-H (1985) Peroxisomal beta-oxidation system of Candida tropicalis . Eur J Biochem 148:285–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

