Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis
- PMID: 25486075
- PMCID: PMC6885065
- DOI: 10.1002/14651858.CD010590.pub2
Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis
Update in
-
Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis.Cochrane Database Syst Rev. 2023 Feb 13;2(2):CD010590. doi: 10.1002/14651858.CD010590.pub3. Cochrane Database Syst Rev. 2023. PMID: 36791280 Free PMC article.
Abstract
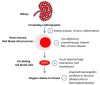
Background: Several erythropoiesis-stimulating agents (ESAs) are available for treating anaemia in people with chronic kidney disease (CKD). Their relative efficacy (preventing blood transfusions and reducing fatigue and breathlessness) and safety (mortality and cardiovascular events) are unclear due to the limited power of head-to-head studies.
Objectives: To compare the efficacy and safety of ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, or methoxy polyethylene glycol-epoetin beta, and biosimilar ESAs, against each other, placebo, or no treatment) to treat anaemia in adults with CKD.
Search methods: We searched the Cochrane Renal Group's Specialised Register to 11 February 2014 through contact with the Trials' Search Co-ordinator using search terms relevant to this review.
Selection criteria: Randomised controlled trials (RCTs) that included a comparison of an ESA (epoetin alfa, epoetin beta, darbepoetin alfa, methoxy polyethylene glycol-epoetin beta, or biosimilar ESA) with another ESA, placebo or no treatment in adults with CKD and that reported prespecified patient-relevant outcomes were considered for inclusion.
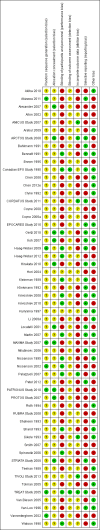
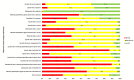
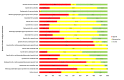
Data collection and analysis: Two independent authors screened the search results and extracted data. Data synthesis was performed by random-effects pairwise meta-analysis and network meta-analysis. We assessed for heterogeneity and inconsistency within meta-analyses using standard techniques and planned subgroup and meta-regression to explore for sources of heterogeneity or inconsistency. We assessed our confidence in treatment estimates for the primary outcomes within network meta-analysis (preventing blood transfusions and all-cause mortality) according to adapted GRADE methodology as very low, low, moderate, or high.
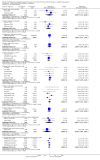
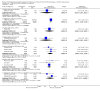
Main results: We identified 56 eligible studies involving 15,596 adults with CKD. Risks of bias in the included studies was generally high or unclear for more than half of studies in all of the risk of bias domains we assessed; no study was low risk for allocation concealment, blinding of outcome assessment and attrition from follow-up. In network analyses, there was moderate to low confidence that epoetin alfa (OR 0.18, 95% CI 0.05 to 0.59), epoetin beta (OR 0.09, 95% CI 0.02 to 0.38), darbepoetin alfa (OR 0.17, 95% CI 0.05 to 0.57), and methoxy polyethylene glycol-epoetin beta (OR 0.15, 95% CI 0.03 to 0.70) prevented blood transfusions compared to placebo. In very low quality evidence, biosimilar ESA therapy was possibly no better than placebo for preventing blood transfusions (OR 0.27, 95% CI 0.05 to 1.47) with considerable imprecision in estimated effects. We could not discern whether all ESAs were similar or different in their effects on preventing blood transfusions and our confidence in the comparative effectiveness of different ESAs was generally very low. Similarly, the comparative effects of ESAs compared with another ESA, placebo or no treatment on all-cause mortality were imprecise.All proprietary ESAs increased the odds of hypertension compared to placebo (epoetin alfa OR 2.31, 95% CI 1.27 to 4.23; epoetin beta OR 2.57, 95% CI 1.23 to 5.39; darbepoetin alfa OR 1.83, 95% CI 1.05 to 3.21; methoxy polyethylene glycol-epoetin beta OR 1.96, 95% CI 0.98 to 3.92), while the effect of biosimilar ESAs on developing hypertension was less certain (OR 1.18, 95% CI 0.47 to 2.99). Our confidence in the comparative effects of ESAs on hypertension was low due to considerable imprecision in treatment estimates. The comparative effects of all ESAs on cardiovascular mortality, myocardial infarction (MI), stroke, and vascular access thrombosis were uncertain and network analyses for major cardiovascular events, end-stage kidney disease (ESKD), fatigue and breathlessness were not possible. Effects of ESAs on fatigue were described heterogeneously in the available studies in ways that were not useable for analyses.
Authors' conclusions: In the CKD setting, there is currently insufficient evidence to suggest the superiority of any ESA formulation based on available safety and efficacy data. Directly comparative data for the effectiveness of different ESA formulations based on patient-centred outcomes (such as quality of life, fatigue, and functional status) are sparse and poorly reported and current research studies are unable to inform care. All proprietary ESAs (epoetin alfa, epoetin beta, darbepoetin alfa, and methoxy polyethylene glycol-epoetin beta) prevent blood transfusions but information for biosimilar ESAs is less conclusive. Comparative treatment effects of different ESA formulations on other patient-important outcomes such as survival, MI, stroke, breathlessness and fatigue are very uncertain.For consumers, clinicians and funders, considerations such as drug cost and availability and preferences for dosing frequency might be considered as the basis for individualising anaemia care due to lack of data for comparative differences in clinical benefits and harms.
Conflict of interest statement
Suetonia C Palmer: none known
Valeria Saglimbene: none known
Dimitris Mavridis: none known
Georgia Salanti: none known
Jonathan C Craig: none known
Marcello Tonelli: Dr Tonelli has received an investigator‐initiated grant and honoraria from Amgen Australia for an academic lecture series ‐‐ neither were related to ESA or anaemia. All honoraria were donated to charity
Natasha Wiebe: none known
Giovanni FM Strippoli: none known
Figures












Comment in
-
Erythropoiesis-stimulating agents for anaemia in chronic kidney disease: Are they all the same?Cochrane Database Syst Rev. 2014 Dec 9;2014(12):ED000093. doi: 10.1002/14651858.ED000093. Cochrane Database Syst Rev. 2014. PMID: 25549989 Free PMC article. No abstract available.
Similar articles
-
Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis.Cochrane Database Syst Rev. 2023 Feb 13;2(2):CD010590. doi: 10.1002/14651858.CD010590.pub3. Cochrane Database Syst Rev. 2023. PMID: 36791280 Free PMC article.
-
Continuous erythropoiesis receptor activator (CERA) for the anaemia of chronic kidney disease.Cochrane Database Syst Rev. 2017 Aug 7;8(8):CD009904. doi: 10.1002/14651858.CD009904.pub2. Cochrane Database Syst Rev. 2017. PMID: 28782299 Free PMC article.
-
Darbepoetin for the anaemia of chronic kidney disease.Cochrane Database Syst Rev. 2014 Mar 31;2014(3):CD009297. doi: 10.1002/14651858.CD009297.pub2. Cochrane Database Syst Rev. 2014. PMID: 24683046 Free PMC article.
-
Short-acting erythropoiesis-stimulating agents for anaemia in predialysis patients.Cochrane Database Syst Rev. 2017 Jan 9;1(1):CD011690. doi: 10.1002/14651858.CD011690.pub2. Cochrane Database Syst Rev. 2017. PMID: 28066881 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
Cited by
-
Mortality risk of darbepoetin alfa versus epoetin alfa in patients with CKD: systematic review and meta-analysis.Am J Kidney Dis. 2015 Jul;66(1):69-74. doi: 10.1053/j.ajkd.2014.12.012. Epub 2015 Jan 28. Am J Kidney Dis. 2015. PMID: 25636816 Free PMC article.
-
Longer-term outcomes of darbepoetin alfa versus epoetin alfa in patients with ESRD initiating hemodialysis: a quasi-experimental cohort study.Am J Kidney Dis. 2015 Jul;66(1):106-13. doi: 10.1053/j.ajkd.2015.02.339. Epub 2015 May 2. Am J Kidney Dis. 2015. PMID: 25943715 Free PMC article.
-
[Austrian Consensus on High Blood Pressure 2019].Wien Klin Wochenschr. 2019 Nov;131(Suppl 6):489-590. doi: 10.1007/s00508-019-01565-0. Wien Klin Wochenschr. 2019. PMID: 31792659 German.
-
Cancer-associated stroke: Pathophysiology, detection and management (Review).Int J Oncol. 2019 Mar;54(3):779-796. doi: 10.3892/ijo.2019.4669. Epub 2019 Jan 2. Int J Oncol. 2019. PMID: 30628661 Free PMC article. Review.
-
Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Non-Dialysis Dependent Chronic Kidney Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials.Indian J Nephrol. 2025 Mar-Apr;35(2):217-233. doi: 10.25259/ijn_382_23. Epub 2025 Feb 25. Indian J Nephrol. 2025. PMID: 40060082 Free PMC article.
References
References to studies included in this review
-
- Akiba T, Akizawa T, Kakuma T. Randomized double‐blind comparative study of recombinant human erythropoietin (epoetin kappa, produced by serum‐free culture) in renal anemia patients on hemodialysis. Japanese Pharmacology & Therapeutics 2010;38(2):181‐98. [EMBASE: 2010186120]
-
- Akaishi M, Hiroe M, Hada Y, Suzuki M, Tsubakihara Y, Akizawa T, et al. Effect of anemia correction on left ventricular hypertrophy in patients with modestly high hemoglobin level and chronic kidney disease. Journal of Cardiology 2013;62(4):249‐56. [MEDLINE: ] - PubMed
- Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Saito A, et al. Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Therapeutic Apheresis & Dialysis 2011;15(5):431‐40. [MEDLINE: ] - PubMed
- Akizawa T, Tsubakihara Y. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the efficacy [abstract no: SU‐PO804]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):762A. [CENTRAL: CN‐00740554]
- Tsubakihara Y, Akizawa T. High target hemoglobin with erythropoiesis stimulating agent (ESA) in chronic kidney disease (CKD) slows the occurrence rate of events related to decline of renal function [abstract no: SA‐FC341]. Journal of the American Society of Nephrology 2009;20:79A. [CENTRAL: CN‐00793981]
- Tsubakihara Y, Akizawa T. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the safety [abstract no: SU‐PO818]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):765A. [CENTRAL: CN‐00740542]
-
- Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Current Medical Research & Opinion 2007;23(12):2997‐3008. [MEDLINE: ] - PubMed
- Thadhani R, Cheriyan R, Brenner R, Ford J, Powers K, Rahman SN. Treatment of anemia with ARANESP (darbepoetin alfa) improves health related quality of life (HRQOL) in patients with chronic kidney disease (CKD) [abstract]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):637a. [CENTRAL: CN‐00447983]
-
- Allon M, Kleinman AK, Walczyk M, Kaupke C, Maroni BJ, Heatherington A, et al. The pharmacokinetics of novel erythropoiesis stimulating protein (NESP) following chronic intravenous administration is time‐and dose‐linear [abstract no: A1308]. Journal of the American Society of Nephrology 2000;11(Sept):248A. [CENTRAL: CN‐00626053]
- Allon M, Kleinman K, Walczyk M, Kaupke C, Messer‐Mann L, Olson K, et al. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clinical Pharmacology & Therapeutics 2002;72(5):546‐55. [MEDLINE: ] - PubMed
-
- Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetic parameters of C.E.R.A. and are not affected by age in patients with chronic kidney disease on dialysis [abstract no: SaP351]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi351. [CENTRAL: CN‐00757500]
- Chanu P, Gieschke R, Reigner B, Dougherty FC. Pharmacokinetics of C.E.R.A. and stable maintenance of haemoglobin (Hb) levels with once‐monthly dosing in patients with chronic kidney disease (CKD) [abstract no: SaP325]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi342. [CENTRAL: CN‐00757502]
- Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571]
- Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, et al. C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered at extended intervals corrects Hb levels in patients with CKD on dialysis [abstract no: SA‐PO212]. Journal of the American Society of Nephrology 2006;17(Abstracts):620A. [CENTRAL: CN‐00644218]
- Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, et al. Efficacy of intravenous methoxy polyethylene glycol‐epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. American Journal of Kidney Diseases 2007;50(6):989‐1000. [MEDLINE: ] - PubMed
- Provenzano R, Macdougall IC, Law A, Ouyang Y, Bexon M. Anemia correction with C.E.R.A. in patients (pts) with chronic kidney disease (CKD) is unaffected by baseline hemoglobin (Hb) level [abstract no: SU‐PO796]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00747311] - PubMed
References to studies excluded from this review
-
- Acchiardo SR, Quinn BP, Moore LW, Burk LB, Miles DE. Evaluation of hemodialysis patients treated with erythropoietin. American Journal of Kidney Diseases 1991;17(3):290‐4. [MEDLINE: ] - PubMed
-
- Laville M, Anaemia CORrection in Diabetes trial. New strategies in anaemia management: ACORD (Anaemia CORrection in Diabetes) trial. Acta Diabetologica 2004;41 Suppl 1:S18‐22. [MEDLINE: ] - PubMed
- Ritz E, Bilous R, O'Donoghue D, Laville M, Alvaro F. Prescription patterns of cardio‐ and reno‐protective agents in early diabetic nephropathy: baseline data from the ACORD trial [abstract no: PUB100]. Journal of the American Society of Nephrology 2004;15(Oct):783A. [CENTRAL: CN‐00550505]
- Ritz E, Bilous RW, Alvaro F, Laville M, O'Donoghue D, Archerhag A. Anemia correction with epoetin beta in patients with diabetes and chronic kidney disease ‐ primary results of the anaemia correction in diabetes (ACORD) study [abstract no: SO022]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv11. [CENTRAL: CN‐00763625]
- Ritz E, Laville M, Bilous R W, O'Donoghue D, Scherhag A, Burger U, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. American Journal of Kidney Diseases 2007;49(2):194‐207. [MEDLINE: ] - PubMed
-
- Francisco AL, Sulowicz W, Dougherty FC. Subcutaneous CERA (continuous erythropoiesis receptor activator) has potent erythropoietic activity in dialysis patients with chronic renal anemia: an exploratory multiple‐dose study [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):27A‐8A. [CENTRAL: CN‐00550366]
- Francisco AL, Sulowicz W, Klinger M, Niemczyk S, Vargemezis V, Metivier F, et al. Continuous Erythropoietin Receptor Activator (C.E.R.A.) administered at extended administration intervals corrects anaemia in patients with chronic kidney disease on dialysis: a randomised, multicentre, multiple‐dose, phase II study. International Journal of Clinical Practice 2006;60(12):1687‐96. [MEDLINE: ] - PubMed
-
- Besarab A, Beyer U, Dougherty FC. Long‐term intravenous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations in hemodialysis patients [abstract no: W‐PO40127]. Nephrology 2005;10(Suppl 1):A312‐3. [CENTRAL: CN‐00747326]
- Besarab A, Salifu MO, Lunde NM, Bansal V, Fishbane S, Dougherty FC, et al. Efficacy and tolerability of intravenous continuous erythropoietin receptor activator: a 19‐week, phase II, multicenter, randomized, open‐label, dose‐finding study with a 12‐month extension phase in patients with chronic renal disease. Clinical Therapeutics 2007;29(4):626‐39. [MEDLINE: ] - PubMed
- Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W‐PO40131]. Nephrology 2005;10(Suppl 1):A313‐4. [CENTRAL: CN‐00602138]
- Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W‐PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN‐00602137]
- Dougherty FC, Loghman‐Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN‐00602143]
- Dutka P, Tilocca P, BA16285 and BA16286 Study Groups. CERA (Continuous Erythropoietin Receptor Activator) maintains stable hemoglobin concentrations in dialysis patients irrespective of gender, age, race or diabetic status [abstract]. Nephrology Nursing Journal 2006;33(2):138. [CENTRAL: CN‐00602144]
-
- Dougherty FC, Beyer U. No changes in blood pressure in dialysis patients after 12 months of treatment with IV/SC CERA (Continuous Erythropoietin Receptor Activator) [abstract no: W‐PO40131]. Nephrology 2005;10(Suppl 1):A313‐4. [CENTRAL: CN‐00602138]
- Dougherty FC, Beyer U. Safety and tolerability profile of continuous erythropoietin receptor activator (CERA) with extended dosing intervals in patients with chronic kidney disease on dialysis [abstract no: W‐PO40130]. Nephrology 2005;10(Suppl 1):A313. [CENTRAL: CN‐00602137]
- Dougherty FC, Loghman‐Adham M, Schultze N, Beyer U. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients regardless of gender, age, race and diabetic status [abstract no: MP206]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v269. [CENTRAL: CN‐00602143]
- Dutka P, Tilocca P, BA16285 and BA16286 Study Groups. CERA (Continuous Erythropoietin Receptor Activator) maintains stable hemoglobin concentrations in dialysis patients irrespective of gender, age, race or diabetic status [abstract]. Nephrology Nursing Journal 2006;33(2):138. [CENTRAL: CN‐00602144]
- Locatelli F, Villa G, Arias M, Marchesi D, Dougherty FC, Beyer U, et al. CERA (Continuous Erythropoietin receptor activator) maintains hemoglobin levels in dialysis patients when administered subcutaneously up to once every 4 weeks. [abstract no: SU‐PO051]. Journal of the American Society of Nephrology 2004;15(Oct):543A. [CENTRAL: CN‐00626008]
- Locatelli F, Villa G, Beyer U, Dougherty FC. Subcutaneous CERA (Continuous Erythropoietin Receptor Activator) maintains hemoglobin concentrations with dosing intervals up to 4 weeks in dialysis patients [abstract no: MP183]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v261. [CN‐00602142]
- Locatelli F, Villa G, Francisco AL, Albertazzi A, Adrogue HJ, Dougherty FC, et al. Effect of a continuous erythropoietin receptor activator (C.E.R.A.) on stable haemoglobin in patients with CKD on dialysis: once monthly administration. Current Medical Research & Opinion 2007;23(5):969‐79. [MEDLINE: ] - PubMed
- Salifu M, Villa G, Dougherty FC. Adequate hemoglobin levels are maintained with continuous erythropoietin receptor activator (CERA) in dialysis patients with different ranges of iron status and pre‐existing conditions. [abstract no: 138]. American Journal of Kidney Diseases 2006;47(4):A53. [CENTRAL: CN‐00602141]
References to studies awaiting assessment
-
- Barany P. Treatment of anemia in hemodialysis (HD) patients (PTS) to a normal hemoglobin concentration (HB) ‐ results of an open randomized clinical trial of epoetin beta [abstract no: M387]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):243A. [CENTRAL: CN‐00444332] - PubMed
-
- Carrera F, Anunciada AI, Nogueira C, Silva JG. Comparison of HB levels in dialysis patients receiving three‐times weekly rHuepo switched to once‐weekly darbepoetin alfa: results of a randomized study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):164. [CENTRAL: CN‐00444694]
-
- Nissenson A, Nassar G, Edwardes M, Beswick R, Berns J. C.E.R.A. maintains hemoglobin in dialysis patients directly switched from epoetin (EPO) without increasing iron therapy requirements [abstract no: F‐PO855]. Journal of the American Society of Nephrology 2007;18(Abstracts):290A‐1A.
-
- Ostrvica E, Mesic E, Ostrvica D, Delic J, Delic‐Custendil S, Hukic F. Effectiveness of treating the renal anemia in chronic hemodialyzed patients by epoietin alpha and beta. Medicinski Arhiv 2010;64(1):4‐6. [MEDLINE: ] - PubMed
-
- Palazzuoli A, Quatrini I, Calabro A, Antonelli G, Caputo M, Campagna MS, et al. Anemia correction by erythropoietin reduces BNP levels, hospitalization rate, and NYHA class in patients with cardio‐renal anemia syndrome. Clinical & Experimental Medicine 2011;11(1):43‐8. [MEDLINE: ] - PubMed
References to ongoing studies
-
- Besarab A, Canaud B, Francisco AL, Kerr P, Locatelli F, Lok CE, et al. Randomized comparison of IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) and Darbepoetin Alfa (DA) at extended administration intervals for the maintenance of Hb levels in patients with CKD on dialysis: rationale and design [abstract no: PUB377]. Journal of the American Society of Nephrology 2006;17(Abstracts):896A. [CENTRAL: CN‐00740564]
-
- NCT00442702. A randomized, open label study to compare the effect of monthly subcutaneous mircera with that of darbepoetin alfa, given according to local label, on the management of anemia in patients with chronic kidney disease not on dialysis. www.clinicaltrials.gov/show/NCT00442702 (accessed 8 October 2014).
-
- NCT00559273. An open‐label, randomized, multicenter, parallel‐group study to demonstrate correction of anemia using once every 4 weeks subcutaneous injections of mircera in patients with chronic kidney disease who are not on dialysis. www.clinicaltrials.gov/ct2/show/NCT00559273 (accessed 8 October 2014).
-
- NCT00717821. A randomized, controlled, open label, French multicenter parallel group study to compare the hemoglobin maintenance with once monthly administration of mircera versus epoetin beta or darbepoetin alfa in patients with chronic kidney disease on hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00717821 (accessed 8 October 2014).
-
- NCT00773513. A randomized, open label study to assess all‐cause mortality and cardiovascular morbidity in patients with chronic kidney disease on dialysis and those not on renal replacement therapy under treatment with mircera or reference ESAs. www.clinicaltrials.gov/ct2/show/NCT00773513 (accessed 8 October 2014).
Additional references
-
- Seidenfeld J, Piper M, Bohlius J, Weingart O, Trelle S, Engert A, et al. Comparative effectiveness of epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment. Comparative effectiveness review No. 3. (Prepared by Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under Contract No. 290‐02‐0026). Rockville, MD: Agency for Healthcare Research and Quality; May 2006. www.effectivehealthcare.ahrq.gov/reports/final.cfm (accessed 8 October 2014).
-
- Grant MD, Piper M, Bohlius J, Tonia T, Robert N, Vats V, et al. Epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment: comparative effectiveness update. Comparative Effectiveness Review No. 113. (Prepared by the Blue Cross and Blue Shield Association Technology Evaluation Center Evidence‐based Practice Center under Contract No. 290‐2007‐10058‐I). AHRQ Publication No. 13‐EHC077‐EF. Rockville, MD: Agency for Healthcare Research and Quality. 2013. http://effectivehealthcare.ahrq.gov/ehc/products/170/1481/cancer‐anemia‐... (accessed 8 October 2014). - PubMed
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

