Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy
- PMID: 25486088
- PMCID: PMC4339497
- DOI: 10.1016/j.preteyeres.2014.11.005
Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy
Abstract
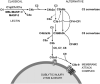
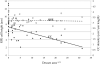
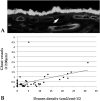
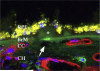
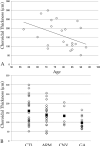
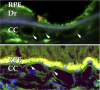
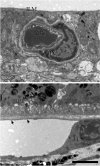
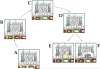
Age-related macular degeneration (AMD) is a common and devastating disease that can result in severe visual dysfunction. Over the last decade, great progress has been made in identifying genetic variants that contribute to AMD, many of which lie in genes involved in the complement cascade. In this review we discuss the significance of complement activation in AMD, particularly with respect to the formation of the membrane attack complex in the aging choriocapillaris. We review the clinical, histological and biochemical data that indicate that vascular loss in the choroid occurs very early in the pathogenesis of AMD, and discuss the potential impact of vascular dropout on the retinal pigment epithelium, Bruch's membrane and the photoreceptor cells. Finally, we present a hypothesis for the pathogenesis of early AMD and consider the implications of this model on the development of new therapies.
Keywords: Age-related macular degeneration; Choriocapillaris; Choroidmacula; Complement system; Endothelial cells; Pathophysiology.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures







References
-
- Abi-Hanna D, Wakefield D, Watkins S. HLA antigens in ocular tissues. I. In vivo expression in human eyes. Transplantation. 1988;45:610–613. - PubMed
-
- Ablonczy Z, Dahrouj M, Marneros AG. Progressive dysfunction of the retinal pigment epithelium and retina due to increased VEGF-A levels. FASEB J. 2014;28:2369–2379. http://dx.doi.org/10.1096/fj.13-248021. - DOI - PMC - PubMed
-
- Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. http://dx.doi.org/10.1001/jama.2013.4997. - DOI - PubMed
-
- Allende A, Madigan MC, Provis JM. Endothelial cell proliferation in the choriocapillaris during human retinal differentiation. Br. J. Ophthalmol. 2006;90:1046–1051. http://dx.doi.org/10.1136/bjo.2006.092080. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

