A mutation in Na(+)-NQR uncouples electron flow from Na(+) translocation in the presence of K(+)
- PMID: 25486106
- PMCID: PMC5019955
- DOI: 10.1021/bi501266e
A mutation in Na(+)-NQR uncouples electron flow from Na(+) translocation in the presence of K(+)
Abstract
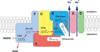
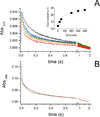
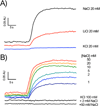
The sodium-pumping NADH:ubiquinone oxidoreductase (Na(+)-NQR) is a bacterial respiratory enzyme that obtains energy from the redox reaction between NADH and ubiquinone and uses this energy to create an electrochemical Na(+) gradient across the cell membrane. A number of acidic residues in transmembrane helices have been shown to be important for Na(+) translocation. One of these, Asp-397 in the NqrB subunit, is a key residue for Na(+) uptake and binding. In this study, we show that when this residue is replaced with asparagine, the enzyme acquires a new sensitivity to K(+); in the mutant, K(+) both activates the redox reaction and uncouples it from the ion translocation reaction. In the wild-type enzyme, Na(+) (or Li(+)) accelerates turnover while K(+) alone does not activate. In the NqrB-D397N mutant, K(+) accelerates the same internal electron transfer step (2Fe-2S → FMNC) that is accelerated by Na(+). This is the same step that is inhibited in mutants in which Na(+) uptake is blocked. NqrB-D397N is able to translocate Na(+) and Li(+), but when K(+) is introduced, no ion translocation is observed, regardless of whether Na(+) or Li(+) is present. Thus, this mutant, when it turns over in the presence of K(+), is the first, and currently the only, example of an uncoupled Na(+)-NQR. The fact the redox reaction and ion pumping become decoupled from each other only in the presence of K(+) provides a switch that promises to be a useful experimental tool.
Figures


References
-
- Dibrov PA, Kostryko VA, Lazarova RL, Skulachev VP, Smirnova IA. The sodium cycle. I. Na+-dependent motility and modes of membrane energization in the marine alkalotolerant Vibrio alginolyticus. Biochim. Biophys. Acta. 1986;850:449–457. - PubMed
-
- Dibrov PA, Lazarova RL, Skulachev VP, Verkhovskaya ML. The sodium cycle. II. Na+-coupled oxidative phosphorylation in Vibrio alginolyticus cells. Biochim. Biophys. Acta. 1986;850:458–465. - PubMed
-
- Terashima H, Kojima S, Homma M. Flagellar motility in bacteria structure and function of flagellar motor. Int. Rev. Cell Mol. Biol. 2008;270:39–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

