Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy
- PMID: 25486131
- PMCID: PMC4339485
- DOI: 10.1053/j.gastro.2014.11.020
Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy
Abstract
Background & aims: Weight loss after pharmacotherapy varies greatly. We aimed to examine associations of quantitative gastrointestinal and psychological traits with obesity, and to validate the ability of these traits to predict responses of obese individuals to pharmacotherapy.
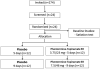
Methods: In a prospective study, we measured gastric emptying of solids and liquids, fasting and postprandial gastric volume, satiation by nutrient drink test (volume to fullness and maximal tolerated volume), satiety after an ad libitum buffet meal, gastrointestinal hormones, and psychological traits in 328 normal-weight, overweight, or obese adults. We also analyzed data from 181 previously studied adults to assess associations betwecen a subset of traits with body mass index and waist circumference. Latent dimensions associated with overweight or obesity were appraised by principal component analyses. We performed a proof of concept, placebo-controlled trial of extended-release phentermine and topiramate in 24 patients to validate associations between quantitative traits and response to weight-loss therapy.
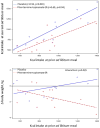
Results: In the prospective study, obesity was associated with fasting gastric volume (P = .03), accelerated gastric emptying (P < .001 for solids and P = .011 for liquids), lower postprandial levels of peptide tyrosine tyrosine (P = .003), and higher postprandial levels of glucagon-like peptide 1 (P < .001). In a combined analysis of data from all studies, obesity was associated with higher volume to fullness (n = 509; P = .038) and satiety with abnormal waist circumference (n = 271; P = .016). Principal component analysis identified latent dimensions that accounted for approximately 81% of the variation among overweight and obese subjects, including satiety or satiation (21%), gastric motility (14%), psychological factors (13%), and gastric sensorimotor factors (11%). The combination of phentermine and topiramate caused significant weight loss, slowed gastric emptying, and decreased calorie intake; weight loss in response to phentermine and topiramate was significantly associated with calorie intake at the prior satiety test.
Conclusions: Quantitative traits are associated with high body mass index; they can distinguish obesity phenotypes and, in a proof of concept clinical trial, predicted response to pharmacotherapy for obesity. ClinicalTrials.gov Number: NCT01834404.
Keywords: BMI; Incretin; Phentermine; Satiety; Topiramate.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Hunger games: is your stomach making you fat?Gastroenterology. 2015 Mar;148(3):491-3. doi: 10.1053/j.gastro.2015.01.010. Epub 2015 Jan 19. Gastroenterology. 2015. PMID: 25613313 No abstract available.
References
-
- Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA : the journal of the American Medical Association. 2012;307:491–497. - PubMed
-
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. - PubMed
-
- Stubbs J, Whybrow S, Teixeira P, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:688–708. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

