Long term outcomes following hospital admission for sepsis using relative survival analysis: a prospective cohort study of 1,092 patients with 5 year follow up
- PMID: 25486241
- PMCID: PMC4259299
- DOI: 10.1371/journal.pone.0112224
Long term outcomes following hospital admission for sepsis using relative survival analysis: a prospective cohort study of 1,092 patients with 5 year follow up
Abstract
Background: Sepsis is a leading cause of death in intensive care units and is increasing in incidence. Current trials of novel therapeutic approaches for sepsis focus on 28-day mortality as the primary outcome measure, but excess mortality may extend well beyond this time period.
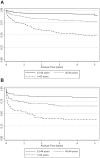
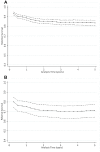
Methods: We used relative survival analysis to examine excess mortality in a cohort of 1,028 patients admitted to a tertiary referral hospital with sepsis during 2007-2008, over the first 5 years of follow up. Expected survival was estimated using the Ederer II method, using Australian life tables as the reference population. Cumulative and interval specific relative survival were estimated by age group, sex, sepsis severity and Indigenous status.
Results: Patients were followed for a median of 4.5 years (range 0-5.2). Of the 1028 patients, the mean age was 46.9 years, 52% were male, 228 (22.2%) had severe sepsis and 218 (21%) died during the follow up period. Mortality based on cumulative relative survival exceeded that of the reference population for the first 2 years post admission in the whole cohort and for the first 3 years in the subgroup with severe sepsis. Independent predictors of mortality over the whole follow up period were male sex, Indigenous Australian ethnicity, older age, higher Charlson Comorbidity Index, and sepsis-related organ dysfunction at presentation.
Conclusions: The mortality rate of patients hospitalised with sepsis exceeds that of the general population until 2 years post admission. Efforts to improve outcomes from sepsis should examine longer term outcomes than the traditional primary endpoints of 28-day and 90-day mortality.
Conflict of interest statement
Figures

References
-
- Alberti C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, et al. (2003) Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med 168:77–84. - PubMed
-
- Angus DC, Pereira CA, Silva E (2006) Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets 6:207–212. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554. - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709. - PubMed
-
- Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, et al. (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. Jama 273:934–941. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

