Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats
- PMID: 25486547
- PMCID: PMC4323744
- DOI: 10.1016/j.nbd.2014.11.024
Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats
Abstract
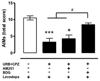
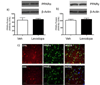
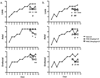
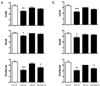
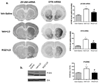
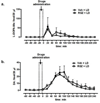
Long-term administration of l-3,4-dihydroxyphenylalanine (levodopa), the mainstay treatment for Parkinson's disease (PD), is accompanied by fluctuations in its duration of action and motor complications (dyskinesia) that dramatically affect the quality of life of patients. Levodopa-induced dyskinesias (LID) can be modeled in rats with unilateral 6-OHDA lesions via chronic administration of levodopa, which causes increasingly severe axial, limb, and orofacial abnormal involuntary movements (AIMs) over time. In previous studies, we showed that the direct activation of CB1 cannabinoid receptors alleviated rat AIMs. Interestingly, elevation of the endocannabinoid anandamide by URB597 (URB), an inhibitor of endocannabinoid catabolism, produced an anti-dyskinetic response that was only partially mediated via CB1 receptors and required the concomitant blockade of transient receptor potential vanilloid type-1 (TRPV1) channels by capsazepine (CPZ) (Morgese et al., 2007). In this study, we showed that the stimulation of peroxisome proliferator-activated receptors (PPAR), a family of transcription factors activated by anandamide, contributes to the anti-dyskinetic effects of URB+CPZ, and that the direct activation of the PPARγ subtype by rosiglitazone (RGZ) alleviates levodopa-induced AIMs in 6-OHDA rats. AIM reduction was associated with an attenuation of levodopa-induced increase of dynorphin, zif-268, and of ERK phosphorylation in the denervated striatum. RGZ treatment did not decrease striatal levodopa and dopamine bioavailability, nor did it affect levodopa anti-parkinsonian activity. Collectively, these data indicate that PPARγ may represent a new pharmacological target for the treatment of LID.
Keywords: Cannabinoid; Dyskinesia; Levodopa; PPARγ; Parkinson's disease; Rosiglitazone.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
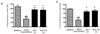
Similar articles
-
The novel 5-HT1A receptor agonist, NLX-112 reduces l-DOPA-induced abnormal involuntary movements in rat: A chronic administration study with microdialysis measurements.Neuropharmacology. 2016 Jun;105:651-660. doi: 10.1016/j.neuropharm.2016.01.013. Epub 2016 Jan 9. Neuropharmacology. 2016. PMID: 26777281
-
Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: Possible mechanism of action.Neurobiol Dis. 2016 Oct;94:179-95. doi: 10.1016/j.nbd.2016.06.013. Epub 2016 Jun 29. Neurobiol Dis. 2016. PMID: 27373843 No abstract available.
-
Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats.Neuropharmacology. 2014 Apr;79:726-37. doi: 10.1016/j.neuropharm.2013.11.024. Epub 2013 Dec 10. Neuropharmacology. 2014. PMID: 24333147
-
Pharmacological options for the management of dyskinesias.Drugs. 1996 Dec;52(6):849-60. doi: 10.2165/00003495-199652060-00006. Drugs. 1996. PMID: 8957155 Review.
-
PPAR-γ: therapeutic prospects in Parkinson's disease.Curr Drug Targets. 2013 Jun;14(7):743-51. doi: 10.2174/1389450111314070004. Curr Drug Targets. 2013. PMID: 23469878 Review.
Cited by
-
Cohort-specific boolean models highlight different regulatory modules during Parkinson's disease progression.iScience. 2024 Sep 14;27(10):110956. doi: 10.1016/j.isci.2024.110956. eCollection 2024 Oct 18. iScience. 2024. PMID: 39429779 Free PMC article.
-
Prevention of L-Dopa-Induced Dyskinesias by MPEP Blockade of Metabotropic Glutamate Receptor 5 Is Associated with Reduced Inflammation in the Brain of Parkinsonian Monkeys.Cells. 2022 Feb 16;11(4):691. doi: 10.3390/cells11040691. Cells. 2022. PMID: 35203338 Free PMC article.
-
The Use of Compounds Derived from Cannabis sativa in the Treatment of Epilepsy, Painful Conditions, and Neuropsychiatric and Neurodegenerative Disorders.Int J Mol Sci. 2024 May 25;25(11):5749. doi: 10.3390/ijms25115749. Int J Mol Sci. 2024. PMID: 38891938 Free PMC article. Review.
-
Nanomedicine to Overcome Current Parkinson's Treatment Liabilities: A Systematic Review.Neurotox Res. 2016 Nov;30(4):715-729. doi: 10.1007/s12640-016-9663-z. Epub 2016 Aug 31. Neurotox Res. 2016. PMID: 27581037
-
From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases.Front Pharmacol. 2020 Mar 6;11:124. doi: 10.3389/fphar.2020.00124. eCollection 2020. Front Pharmacol. 2020. PMID: 32210795 Free PMC article. Review.
References
-
- Graybiel AM, et al. The basal ganglia and adaptive motor control. Science. 1994;265(5180):1826–1831. - PubMed
-
- Rascol O, et al. Limitations of current Parkinson's disease therapy. Ann Neurol. 2003;53(Suppl 3):S3–S12. discussion S12–5. - PubMed
-
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain. 2000;123(11):2297–2305. - PubMed
-
- Bonifati V, et al. Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol. 1994;17(1):73–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

