The Fanconi anemia ID2 complex: dueling saxes at the crossroads
- PMID: 25486561
- PMCID: PMC4612647
- DOI: 10.4161/15384101.2014.956475
The Fanconi anemia ID2 complex: dueling saxes at the crossroads
Abstract
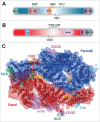
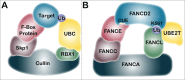
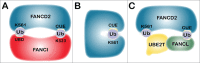
Fanconi anemia (FA) is a rare recessive genetic disease characterized by congenital abnormalities, bone marrow failure and heightened cancer susceptibility in early adulthood. FA is caused by biallelic germ-line mutation of any one of 16 genes. While several functions for the FA proteins have been ascribed, the prevailing hypothesis is that the FA proteins function cooperatively in the FA-BRCA pathway to repair damaged DNA. A pivotal step in the activation of the FA-BRCA pathway is the monoubiquitination of the FANCD2 and FANCI proteins. Despite their importance for DNA repair, the domain structure, regulation, and function of FANCD2 and FANCI remain poorly understood. In this review, we provide an overview of our current understanding of FANCD2 and FANCI, with an emphasis on their posttranslational modification and common and unique functions.
Keywords: AML , acute myeloid leukemia; APC/C, anaphase-promoting complex/cyclosome; APH, aphidicolin; ARM, armadillo repeat domain; AT, ataxia-telangiectasia; ATM, ataxia-telangiectasia mutated; ATR, ATM and Rad3-related; BAC, bacterial-artificial-chromosome; BS, Bloom syndrome; CUE, coupling of ubiquitin conjugation to endoplasmic reticulum degradation; ChIP-seq, CHIP sequencing; CtBP, C-terminal binding protein; CtIP, CtBP-interacting protein; DNA interstrand crosslink repair; DNA repair; EPS15, epidermal growth factor receptor pathway substrate 15; FA, Fanconi anemia; FAN1, FANCD2-associated nuclease1; FANCD2; FANCI; FISH, fluorescence in situ hybridization; Fanconi anemia; HECT, homologous to E6-AP Carboxy Terminus; HJ, Holliday junction; HR, homologous recombination; MCM2-MCM7, minichromosome maintenance 2–7; MEFs, mouse embryonic fibroblasts; MMC, mitomycin C; MRN, MRE11/RAD50/NBS1; NLS, nuclear localization signal; PCNA, proliferating cell nuclear antigen; PIKK, phosphatidylinositol-3-OH-kinase-like family of protein kinases; PIP-box, PCNA-interacting protein motif; POL κ, DNA polymerase κ; RACE, rapid amplification of cDNA ends; RING, really interesting new gene; RTK, receptor tyrosine kinase; SCF, Skp1/Cullin/F-box protein complex; SCKL1, seckel syndrome; SILAC, stable isotope labeling with amino acids in cell culture; SLD1/SLD2, SUMO-like domains; SLIM, SUMO-like domain interacting motif; TIP60, 60 kDa Tat-interactive protein; TLS, Translesion DNA synthesis; UAF1, USP1-associated factor 1; UBD, ubiquitin-binding domain; UBZ, ubiquitin-binding zinc finger; UFB, ultra-fine DNA bridges; UIM, ubiquitin-interacting motif; ULD, ubiquitin-like domain; USP1, ubiquitin-specific protease 1; VRR-nuc, virus-type replication repair nuclease; iPOND, isolation of proteins on nascent DNA; ubiquitin.
Figures
References
-
- Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, Thayer M, Cox B, Olson S, D'Andrea AD, et al. . Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol Cell 2001; 7:241-8; PMID:11239453; http://dx.doi.org/10.1016/S1097-2765(01)00172-1 - DOI - PubMed
-
- Hejna JA, Timmers CD, Reifsteck C, Bruun DA, Lucas LW, Jakobs PM, Toth-Fejel S, Unsworth N, Clemens SL, Garcia DK, et al. . Localization of the Fanconi anemia complementation group D gene to a 200-kb region on chromosome 3p25.3. Am J Hum Genet 2000; 66:1540-51; PMID:10762542; http://dx.doi.org/10.1086/302896 - DOI - PMC - PubMed
-
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D'Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell 2001; 7:249-62; PMID:11239454; http://dx.doi.org/10.1016/S1097-2765(01)00173-3 - DOI - PubMed
-
- Whitney M, Thayer M, Reifsteck C, Olson S, Smith L, Jakobs PM, Leach R, Naylor S, Joenje H, Grompe M. Microcell mediated chromosome transfer maps the Fanconi anaemia group D gene to chromosome 3p. Nat Genet 1995; 11:341-3; PMID:7581463; http://dx.doi.org/10.1038/ng1195-341 - DOI - PubMed
-
- Jakobs PM, Fiddler-Odell E, Reifsteck C, Olson S, Moses RE, Grompe M. Complementation group assignments in Fanconi anemia fibroblast cell lines from North America. Somat Cell Mol Genet 1997; 23:1-7; PMID:9217996; http://dx.doi.org/10.1007/BF02679950 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
