Effectiveness of different Web-based interventions to prepare co-smokers of cigarettes and cannabis for double cessation: a three-arm randomized controlled trial
- PMID: 25486674
- PMCID: PMC4275498
- DOI: 10.2196/jmir.3246
Effectiveness of different Web-based interventions to prepare co-smokers of cigarettes and cannabis for double cessation: a three-arm randomized controlled trial
Abstract
Background: The relationship between tobacco and cannabis use is strong. When co-smokers try to quit only one substance, this relationship often leads to a substitution effect, that is, the increased use of the remaining substance. Stopping the use of both substances simultaneously is therefore a reasonable strategy, but co-smokers rarely report feeling ready for simultaneous cessation. Thus, the question of how co-smokers can be motivated to attempt a simultaneous cessation has arisen. To reach as many co-smokers as possible, we developed brief Web-based interventions aimed at enhancing the readiness to simultaneously quit tobacco and cannabis use.
Objective: Our aim was to analyze the efficacy of three different Web-based interventions designed to enhance co-smokers' readiness to stop tobacco and cannabis use simultaneously.
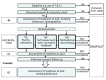
Methods: Within a randomized trial, three brief Web-based and fully automated interventions were compared. The first intervention combined the assessment of cigarette dependence and problematic cannabis use with personalized, normative feedback. The second intervention was based on principles of motivational interviewing. As an active psychoeducational control group, the third intervention merely provided information on tobacco, cannabis, and the co-use of the two substances. The readiness to quit tobacco and cannabis simultaneously was measured before and after the intervention (both online) and 8 weeks later (online or over the phone). Secondary outcomes included the frequency of cigarette and cannabis use, as measured at baseline and after 8 weeks.
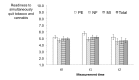
Results: A total of 2467 website users were assessed for eligibility based on their self-reported tobacco and cannabis co-use, and 325 participants were ultimately randomized and analyzed. For the post-intervention assessment, generalized estimating equations revealed a significant increase in the readiness to quit tobacco and cannabis in the total sample (B=.33, 95% CI 0.10-0.56, P=.006). However, this effect was not significant for the comparison between baseline and the 8-week follow-up assessment (P=.69). Furthermore, no differential effects between the interventions were found, nor were any significant intervention or time effects found on the frequency of tobacco or cannabis use.
Conclusions: In the new field of dual interventions for co-smokers of tobacco and cannabis, Web-based interventions can increase the short-term readiness to quit tobacco and cannabis simultaneously. The studied personalized techniques were no more effective than was psychoeducation. The analyzed brief interventions did not change the secondary outcomes, that is the frequency of tobacco and cannabis use.
Trial registration: International Standard Randomized Controlled Trial Number (ISRCTN): 56326375; http://www.isrctn.com/ISRCTN56326375 (Archived by WebCite at http://www.webcitation.org/6UUWBh8u0).
Keywords: cannabis; co-smoking; motivational enhancement; motivational interviewing; personalized feedback; simultaneous cessation; tobacco; web-based intervention.
Conflict of interest statement
Conflicts of Interest: The authors were involved in the development of the interventions.
Figures
References
-
- World Health Organization WHO report on the global tobacco epidemic: warning about the dangers of tobacco. 2011. [2014-01-13]. http://whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf.
-
- UNODC World drug report. 2012. [2014-01-13]. http://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_sm....
-
- Caldeira KM, O'Grady KE, Vincent KB, Arria AM. Marijuana use trajectories during the post-college transition: health outcomes in young adulthood. Drug Alcohol Depend. 2012 Oct 1;125(3):267–75. doi: 10.1016/j.drugalcdep.2012.02.022. http://europepmc.org/abstract/MED/22464050 - DOI - PMC - PubMed
-
- Richter KP, Kaur H, Resnicow K, Nazir N, Mosier MC, Ahluwalia JS. Cigarette smoking among marijuana users in the United States. Subst Abus. 2004 Jun;25(2):35–43. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

