Novel Lys63-linked ubiquitination of IKKβ induces STAT3 signaling
- PMID: 25486864
- PMCID: PMC4615003
- DOI: 10.4161/15384101.2014.988026
Novel Lys63-linked ubiquitination of IKKβ induces STAT3 signaling
Abstract
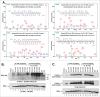
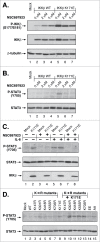
NFκB signaling plays a significant role in human disease, including breast and ovarian carcinoma, insulin resistance, embryonic lethality and liver degeneration, rheumatoid arthritis, aging and Multiple Myeloma (MM). Inhibitor of κB (IκB) kinase β (IKKβ) regulates canonical Nuclear Factor κB (NFκB) signaling in response to inflammation and cellular stresses. NFκB activation requires Lys63-linked (K63-linked) ubiquitination of upstream proteins such as NEMO or TAK1, forming molecular complexes with membrane-bound receptors. We demonstrate that IKKβ itself undergoes K63-linked ubiquitination. Mutations in IKKβ at Lys171, identified in Multiple Myeloma and other cancers, lead to a dramatic increase in kinase activation and K63-linked ubiquitination. These mutations also result in persistent activation of STAT3 signaling. Liquid chromatography (LC)-high mass accuracy tandem mass spectrometry (MS/MS) analysis identified Lys147, Lys418, Lys555 and Lys703 as predominant ubiquitination sites in IKKβ. Specific inhibition of the UBC13-UEV1A complex responsible for K63-linked ubiquitination establishes Lys147 as the predominant site of K63-ubiquitin conjugation and responsible for STAT3 activation. Thus, IKKβ activation leads to ubiquitination within the kinase domain and assemblage of a K63-ubiquitin conjugated signaling platform. These results are discussed with respect to the importance of upregulated NFκB signaling known to occur frequently in MM and other cancers.
Keywords: IKKβ; K-63 ubiquitination; NFκB; STAT3; cell proliferation; inflammatory signaling; mass spectrometry; multiple myeloma; oncogenesis; polyubiquitination.
Figures



Comment in
-
EcSTAT3ic about K63-Linked Ubiquitylation of IKKβ.Cell Cycle. 2015;14(11):1621-2. doi: 10.1080/15384101.2015.1032651. Cell Cycle. 2015. PMID: 25928302 Free PMC article. No abstract available.
References
-
- Hernandez L, Hsu SC, Davidson B, Birrer MJ, Kohn EC, Annunziata CM. Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer. Cancer Res 2010; 70:4005-14; PMID:20424119; http://dx.doi.org/10.1158/0008-5472.CAN-09-3912 - DOI - PMC - PubMed
-
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. . IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004; 117:225-37; PMID:15084260; http://dx.doi.org/10.1016/S0092-8674(04)00302-2 - DOI - PubMed
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11:191-8; PMID:15685170; http://dx.doi.org/10.1038/nm1185 - DOI - PubMed
-
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005; 11:183-90; PMID:15685173; http://dx.doi.org/10.1038/nm1166 - DOI - PMC - PubMed
-
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity 1999; 10:421-9; PMID:10229185; http://dx.doi.org/10.1016/S1074-7613(00)80042-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
