Time course of spinal doublecortin expression in developing rat and porcine spinal cord: implication in in vivo neural precursor grafting studies
- PMID: 25487013
- PMCID: PMC11486198
- DOI: 10.1007/s10571-014-0145-7
Time course of spinal doublecortin expression in developing rat and porcine spinal cord: implication in in vivo neural precursor grafting studies
Abstract
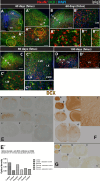
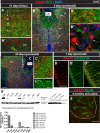
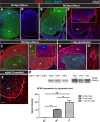
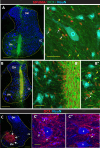
Expression of doublecortin (DCX), a 43-53 kDa microtubule binding protein, is frequently used as (i) an early neuronal marker to identify the stage of neuronal maturation of in vivo grafted neuronal precursors (NSCs), and (ii) a neuronal fate marker transiently expressed by immature neurons during development. Reliable identification of the origin of DCX-immunoreactive cells (i.e., host vs. graft) requires detailed spatial and temporal mapping of endogenous DCX expression at graft-targeted brain or spinal cord regions. Accordingly, in the present study, we analyzed (i) the time course of DCX expression in pre- and postnatal rat and porcine spinal cord, and (ii) the DCX expression in spinally grafted porcine-induced pluripotent stem cells (iPS)-derived NSCs and human embryonic stem cell (ES)-derived NSCs. In addition, complementary temporospatial GFAP expression study in porcine spinal cord was also performed. In 21-day-old rat fetuses, an intense DCX immunoreactivity distributed between the dorsal horn (DH) and ventral horn was seen and was still present in the DH neurons on postnatal day 20. In animals older than 8 weeks, no DCX immunoreactivity was seen at any spinal cord laminae. In contrast to rat, in porcine spinal cord (gestational period 113-114 days), DCX was only expressed during the pre-natal period (up to 100 days) but was no longer present in newborn piglets or in adult animals. Immunohistochemical analysis was confirmed with a comparable expression profile by western blot analysis. Contrary, the expression of porcine GFAP started within 70-80 days of the pre-natal period. Spinally grafted porcine iPS-NSCs and human ES-NSCs showed clear DCX expression at 3-4 weeks postgrafting. These data indicate that in spinal grafting studies which employ postnatal or adult porcine models, the expression of DCX can be used as a reliable marker of grafted neurons. In contrast, if grafted neurons are to be analyzed during the first 4 postnatal weeks in the rat spinal cord, additional markers or grafted cell-specific labeling techniques need to be employed to reliably identify grafted early postmitotic neurons and to differentiate the DCX expression from the neurons of the host.
Figures
References
-
- Couillard-Despres S, Finkl R, Winner B, Ploetz S, Wiedermann D, Aigner R, Bogdahn U, Winkler J, Hoehn M, Aigner L (2008) In vivo optical imaging of neurogenesis: watching new neurons in the intact brain. Mol Imaging 7(1):28–34 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

