Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells
- PMID: 25487152
- PMCID: PMC4336218
- DOI: 10.1038/nature13981
Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells
Abstract
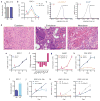
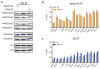
The role of cellular metabolism in regulating cell proliferation and differentiation remains poorly understood. For example, most mammalian cells cannot proliferate without exogenous glutamine supplementation even though glutamine is a non-essential amino acid. Here we show that mouse embryonic stem (ES) cells grown under conditions that maintain naive pluripotency are capable of proliferation in the absence of exogenous glutamine. Despite this, ES cells consume high levels of exogenous glutamine when the metabolite is available. In comparison to more differentiated cells, naive ES cells utilize both glucose and glutamine catabolism to maintain a high level of intracellular α-ketoglutarate (αKG). Consequently, naive ES cells exhibit an elevated αKG to succinate ratio that promotes histone/DNA demethylation and maintains pluripotency. Direct manipulation of the intracellular αKG/succinate ratio is sufficient to regulate multiple chromatin modifications, including H3K27me3 and ten-eleven translocation (Tet)-dependent DNA demethylation, which contribute to the regulation of pluripotency-associated gene expression. In vitro, supplementation with cell-permeable αKG directly supports ES-cell self-renewal while cell-permeable succinate promotes differentiation. This work reveals that intracellular αKG/succinate levels can contribute to the maintenance of cellular identity and have a mechanistic role in the transcriptional and epigenetic state of stem cells.
Figures
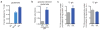

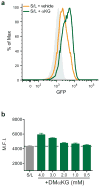

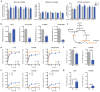
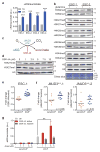

References
-
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. - PubMed
-
- Eagle H, Oyama VI, Levy M, Horton CL, Fleischman R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J Biol Chem. 1956;218:607–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

