Molecular architecture and function of the hemidesmosome
- PMID: 25487405
- PMCID: PMC4544487
- DOI: 10.1007/s00441-014-2061-z
Molecular architecture and function of the hemidesmosome
Abstract
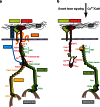
Hemidesmosomes are multiprotein complexes that facilitate the stable adhesion of basal epithelial cells to the underlying basement membrane. The mechanical stability of hemidesmosomes relies on multiple interactions of a few protein components that form a membrane-embedded tightly-ordered complex. The core of this complex is provided by integrin α6β4 and P1a, an isoform of the cytoskeletal linker protein plectin that is specifically associated with hemidesmosomes. Integrin α6β4 binds to the extracellular matrix protein laminin-332, whereas P1a forms a bridge to the cytoplasmic keratin intermediate filament network. Other important components are BPAG1e, the epithelial isoform of bullous pemphigoid antigen 1, BPAG2, a collagen-type transmembrane protein and CD151. Inherited or acquired diseases in which essential components of the hemidesmosome are missing or structurally altered result in tissue fragility and blistering. Modulation of hemidesmosome function is of crucial importance for a variety of biological processes, such as terminal differentiation of basal keratinocytes and keratinocyte migration during wound healing and carcinoma invasion. Here, we review the molecular characteristics of the proteins that make up the hemidesmosome core structure and summarize the current knowledge about how their assembly and turnover are regulated by transcriptional and post-translational mechanisms.
Figures


References
-
- Ackerl R, Walko G, Fuchs P, Fischer I, Schmuth M, Wiche G. Conditional targeting of plectin in prenatal and adult mouse stratified epithelia causes keratinocyte fragility and lesional epidermal barrier defects. J Cell Sci. 2007;120:2435–2443. - PubMed
-
- Alt A, Ohba M, Li L, Gartsbein M, Belanger A, Denning MF, Kuroki T, Yuspa SH, Tennenbaum T. Protein kinase Cdelta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res. 2001;61:4591–4598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

