UV exposure modulates hemidesmosome plasticity, contributing to long-term pigmentation in human skin
- PMID: 25488118
- PMCID: PMC4398603
- DOI: 10.1002/path.4497
UV exposure modulates hemidesmosome plasticity, contributing to long-term pigmentation in human skin
Abstract
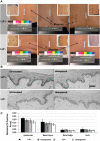
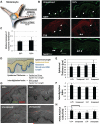
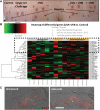
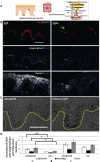
Human skin colour, ie pigmentation, differs widely among individuals, as do their responses to various types of ultraviolet radiation (UV) and their risks of skin cancer. In some individuals, UV-induced pigmentation persists for months to years in a phenomenon termed long-lasting pigmentation (LLP). It is unclear whether LLP is an indicator of potential risk for skin cancer. LLP seems to have similar features to other forms of hyperpigmentation, eg solar lentigines or age spots, which are clinical markers of photodamage and risk factors for precancerous lesions. To investigate what UV-induced molecular changes may persist in individuals with LLP, clinical specimens from non-sunburn-inducing repeated UV exposures (UVA, UVB or UVA + UVB) at 4 months post-exposure (short-term LLP) were evaluated by microarray analysis and dataset mining. Validated targets were further evaluated in clinical specimens from six healthy individuals (three LLP+ and three LLP-) followed for more than 9 months (long-term LLP) who initially received a single sunburn-inducing UVA + UVB exposure. The results support a UV-induced hyperpigmentation model in which basal keratinocytes have an impaired ability to remove melanin that leads to a compensatory mechanism by neighbouring keratinocytes with increased proliferative capacity to maintain skin homeostasis. The attenuated expression of SOX7 and other hemidesmosomal components (integrin α6β4 and plectin) leads to increased melanosome uptake by keratinocytes and points to a spatial regulation within the epidermis. The reduced density of hemidesmosomes provides supporting evidence for plasticity at the epidermal-dermal junction. Altered hemidesmosome plasticity, and the sustained nature of LLP, may be mediated by the role of SOX7 in basal keratinocytes. The long-term sustained subtle changes detected are modest, but sufficient to create dramatic visual differences in skin colour. These results suggest that the hyperpigmentation phenomenon leading to increased interdigitation develops in order to maintain normal skin homeostasis in individuals with LLP.
Keywords: hemidesmosome; pigmentation; skin; sunburn; ultraviolet radiation.
Copyright © 2014 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures


Similar articles
-
Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB.J Invest Dermatol. 2010 Jun;130(6):1685-96. doi: 10.1038/jid.2010.5. Epub 2010 Feb 11. J Invest Dermatol. 2010. PMID: 20147966 Free PMC article.
-
Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure.Pigment Cell Res. 2001 Oct;14(5):348-55. doi: 10.1034/j.1600-0749.2001.140506.x. Pigment Cell Res. 2001. PMID: 11601656
-
Keratinocytes control the proliferation and differentiation of cultured epidermal melanocytes from ultraviolet radiation B-induced pigmented spots in the dorsal skin of hairless mice.Pigment Cell Res. 2002 Oct;15(5):391-9. doi: 10.1034/j.1600-0749.2002.02052.x. Pigment Cell Res. 2002. PMID: 12213097
-
Cutaneous solar ultraviolet exposure and clinical aspects of photodamage.Indian J Dermatol Venereol Leprol. 2012 Jun;78 Suppl 1:S9-S14. doi: 10.4103/0378-6323.97350. Indian J Dermatol Venereol Leprol. 2012. PMID: 22710112 Review.
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
Cited by
-
A Genomic Approach to Identify the Different between Acute and Chronic UVB Exposures in the Causation of Inflammation and Cancer.J Cancer Prev. 2022 Dec 31;27(4):199-207. doi: 10.15430/JCP.2022.27.4.199. J Cancer Prev. 2022. PMID: 36713944 Free PMC article. Review.
-
Role of the p53‑TRPM1/miR‑211‑MMP9 axis in UVB‑induced human melanocyte migration and its potential in repigmentation.Int J Mol Med. 2020 Apr;45(4):1017-1026. doi: 10.3892/ijmm.2020.4478. Epub 2020 Jan 27. Int J Mol Med. 2020. PMID: 31985026 Free PMC article.
-
Potential molecular characteristics in situ in response to repetitive UVB irradiation.Diagn Pathol. 2016 Nov 10;11(1):129. doi: 10.1186/s13000-016-0579-y. Diagn Pathol. 2016. PMID: 27829444 Free PMC article.
-
Establishment and validation of evaluation models for post-inflammatory pigmentation abnormalities.Front Immunol. 2022 Oct 27;13:991594. doi: 10.3389/fimmu.2022.991594. eCollection 2022. Front Immunol. 2022. PMID: 36389813 Free PMC article.
-
Understanding the ultrastructural aspects of berberine-induced skin-darkening activity in the toad, Bufo melanostictus, melanophores.J Microsc Ultrastruct. 2015 Oct-Dec;3(4):210-219. doi: 10.1016/j.jmau.2015.07.001. Epub 2015 Aug 5. J Microsc Ultrastruct. 2015. PMID: 30023201 Free PMC article.
References
-
- Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol. 2012;226:158–71. - PubMed
-
- Sherratt MJ, Bayley CP, Reilly SM, et al. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J Pathol. 2010;222:32–40. - PubMed
-
- Bastiaens M, Hoefnagel J, Westendorp R, et al. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17:225–9. - PubMed
-
- Coelho SG, Zhou Y-C, Bushar HF, et al. Insights into UV-induced long-lasting pigmentation (LLP) in human skin. In: Jimbow K, editor. Proc. XXth Intl. Pigment Cell Conf. Medimond; Bologna, Italy: 2008. pp. 137–41.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

