Preliminary study on carprofen concentration measurements after transcutaneous treatment with Vetdrop® in a microfracture joint defect model in sheep
- PMID: 25488522
- PMCID: PMC4263071
- DOI: 10.1186/s12917-014-0268-6
Preliminary study on carprofen concentration measurements after transcutaneous treatment with Vetdrop® in a microfracture joint defect model in sheep
Abstract
Background: The present preliminary study describes concentration time courses of the NSAID carprofen in the plasma and synovial fluid in a microfrature sheep model after transcutaneous treatments with a novel application device (Vetdrop®). To treat circumscribed inflammatory processes a transcutaneous application device could potentially be beneficial. After transcutaneous application normally lower systemic concentrations are measured which may reduce the incidence of side effects, whereas efficacy is still maintained. In this study carprofen was used based on its capacity to provide analgesia after orthopaedic procedures in sheep and it is considered that it may have a positive influence on the healing of cartilage in low concentrations.
Results: In all transcutaneously treated animals, carprofen plasma concentrations exceeded those of synovial fluid, although plasma levels remained significantly reduced (300-fold) as compared to carprofen administered intravenously. Furthermore, in contrast to the intravenously treated animals, a modest accumulation of carprofen in plasma and synovial fluid was observed in the transcutaneously treated animals over the 6-week treatment period.
Conclusions: The transcutaneously administered carprofen using the Vetdrop® device penetrated the skin and both, plasma- and synovial concentrations could be measured repeatedly over time. This novel device may be considered a valuable transcutaneous drug delivery system.
Figures
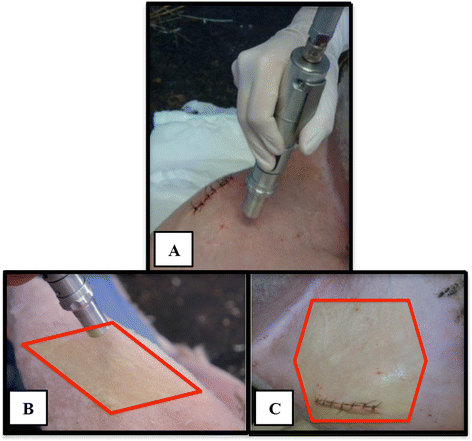
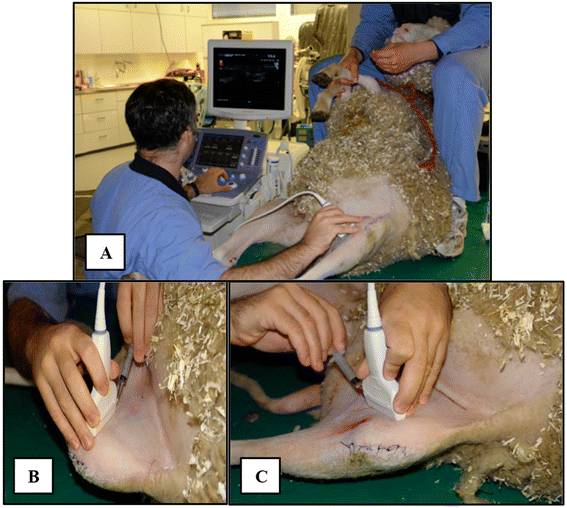
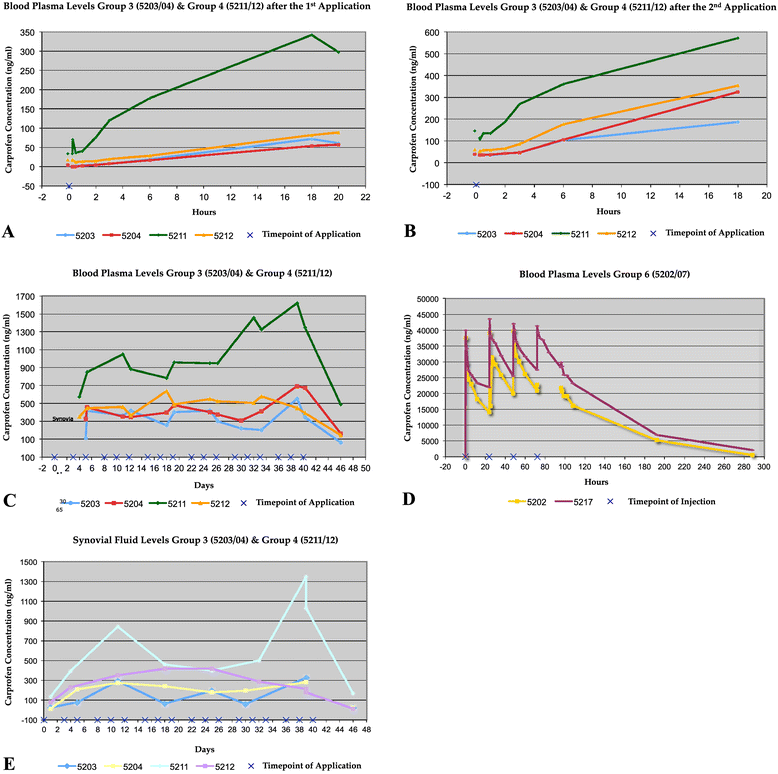
Similar articles
-
Pharmacokinetics of carprofen enantiomers in equine plasma and synovial fluid - a comparison with ketoprofen.J Vet Pharmacol Ther. 1999 Jun;22(3):196-201. doi: 10.1046/j.1365-2885.1999.00202.x. J Vet Pharmacol Ther. 1999. PMID: 10447830
-
Randomised trial of the bioavailability and efficacy of orally administered flunixin, carprofen and ketoprofen in a pain model in sheep.Aust Vet J. 2015 Aug;93(8):265-70. doi: 10.1111/avj.12351. Aust Vet J. 2015. PMID: 26220318 Clinical Trial.
-
Pharmacokinetics of carprofen administered intravenously to sheep.Res Vet Sci. 1992 Sep;53(2):264-6. doi: 10.1016/0034-5288(92)90123-j. Res Vet Sci. 1992. PMID: 1439219
-
Transcutaneous treatment with vetdrop(®) sustains the adjacent cartilage in a microfracturing joint defect model in sheep.Open Orthop J. 2013;7:57-66. doi: 10.2174/1874325001307010057. Epub 2013 Mar 5. Open Orthop J. 2013. PMID: 23539664 Free PMC article.
-
Influence of oxytetracycline on carprofen pharmacodynamics and pharmacokinetics in calves.J Vet Pharmacol Ther. 2013 Aug;36(4):320-8. doi: 10.1111/jvp.12000. Epub 2012 Aug 23. J Vet Pharmacol Ther. 2013. PMID: 22913421 Clinical Trial.
Cited by
-
Analgesia for Sheep in Commercial Production: Where to Next?Animals (Basel). 2021 Apr 14;11(4):1127. doi: 10.3390/ani11041127. Animals (Basel). 2021. PMID: 33920025 Free PMC article. Review.
-
Optimization of a rapid one-step platelet-rich plasma preparation method using syringe centrifugation with and without carprofen.BMC Vet Res. 2020 May 6;16(1):124. doi: 10.1186/s12917-020-02350-2. BMC Vet Res. 2020. PMID: 32375782 Free PMC article.
-
Effect of an Oxygen-Based Mechanical Drug Delivery System on Percutaneous Permeation of Various Substances In Vitro.Pharmaceutics. 2022 Dec 5;14(12):2722. doi: 10.3390/pharmaceutics14122722. Pharmaceutics. 2022. PMID: 36559216 Free PMC article.
-
Swine as the Animal Model for Testing New Formulations of Anti-Inflammatory Drugs: Carprofen Pharmacokinetics and Bioavailability of the Intramuscular Route.Pharmaceutics. 2022 May 12;14(5):1045. doi: 10.3390/pharmaceutics14051045. Pharmaceutics. 2022. PMID: 35631631 Free PMC article.
-
Potent drug delivery enhancement of betulinic acid and NVX-207 into equine skin in vitro - a comparison between a novel oxygen flow-assisted transdermal application device and microemulsion gels.BMC Vet Res. 2024 May 16;20(1):202. doi: 10.1186/s12917-024-04064-1. BMC Vet Res. 2024. PMID: 38755639 Free PMC article.
References
-
- Frey HH, Löscher W. Lehrbuch der Pharmakologie und Toxikologie für die Veterinärmedizin Vol. 3. 2009.
-
- Rietbrock, Staib, and Loew, Klinische Pharmakologie Vol. 4. 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

