Transcription factors GATA4 and HNF4A control distinct aspects of intestinal homeostasis in conjunction with transcription factor CDX2
- PMID: 25488664
- PMCID: PMC4340426
- DOI: 10.1074/jbc.M114.620211
Transcription factors GATA4 and HNF4A control distinct aspects of intestinal homeostasis in conjunction with transcription factor CDX2
Abstract
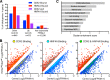
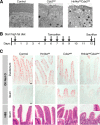
Distinct groups of transcription factors (TFs) assemble at tissue-specific cis-regulatory sites, implying that different TF combinations may control different genes and cellular functions. Within such combinations, TFs that specify or maintain a lineage and are therefore considered master regulators may play a key role. Gene enhancers often attract these tissue-restricted TFs, as well as TFs that are expressed more broadly. However, the contributions of the individual TFs to combinatorial regulatory activity have not been examined critically in many cases in vivo. We address this question using a genetic approach in mice to inactivate the intestine-specifying and intestine-restricted factor CDX2 alone or in combination with its more broadly expressed partner factors, GATA4 and HNF4A. Compared with single mutants, each combination produced significantly greater defects and rapid lethality through distinct anomalies. Intestines lacking Gata4 and Cdx2 were deficient in crypt cell replication, whereas combined loss of Hnf4a and Cdx2 specifically impaired viability and maturation of villus enterocytes. Integrated analysis of TF binding and of transcripts affected in Hnf4a;Cdx2 compound-mutant intestines indicated that this TF pair controls genes required to construct the apical brush border and absorb nutrients, including dietary lipids. This study thus defines combinatorial TF activities, their specific requirements during tissue homeostasis, and modules of transcriptional targets in intestinal epithelial cells in vivo.
Keywords: CDX2 Transcription Factor; Cell Differentiation; Cell Proliferation; GATA Transcription Factor; Gene Regulation; HNF4A Transcription Factor; Intestinal Epithelium; Lipid Metabolism.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding.Mol Cell Biol. 2013 Jan;33(2):281-92. doi: 10.1128/MCB.01185-12. Epub 2012 Nov 5. Mol Cell Biol. 2013. PMID: 23129810 Free PMC article.
-
Cooperation between HNF-1alpha, Cdx2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line.Am J Physiol Gastrointest Liver Physiol. 2010 Apr;298(4):G504-17. doi: 10.1152/ajpgi.00265.2009. Epub 2010 Feb 4. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20133952 Free PMC article.
-
Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene.J Biol Chem. 2002 Aug 30;277(35):31909-17. doi: 10.1074/jbc.M204622200. Epub 2002 Jun 11. J Biol Chem. 2002. PMID: 12060663
-
Transcriptional regulation of metabolism in the intestinal epithelium.Am J Physiol Gastrointest Liver Physiol. 2023 Dec 1;325(6):G501-G507. doi: 10.1152/ajpgi.00147.2023. Epub 2023 Oct 3. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37786942 Free PMC article. Review.
-
Chromatin Dynamics in Intestinal Epithelial Homeostasis: A Paradigm of Cell Fate Determination versus Cell Plasticity.Stem Cell Rev Rep. 2020 Dec;16(6):1062-1080. doi: 10.1007/s12015-020-10055-0. Epub 2020 Oct 13. Stem Cell Rev Rep. 2020. PMID: 33051755 Free PMC article. Review.
Cited by
-
PepT1 Expression Helps Maintain Intestinal Homeostasis by Mediating the Differential Expression of miRNAs along the Crypt-Villus Axis.Sci Rep. 2016 Jun 1;6:27119. doi: 10.1038/srep27119. Sci Rep. 2016. PMID: 27250880 Free PMC article.
-
Src family kinases inhibit differentiation of intestinal epithelial cells through the Hippo effector YAP1.Biol Open. 2021 Nov 15;10(11):bio058904. doi: 10.1242/bio.058904. Epub 2021 Nov 15. Biol Open. 2021. PMID: 34693980 Free PMC article.
-
Analysis of the HNF4A isoform-regulated transcriptome identifies CCL15 as a downstream target in gastric carcinogenesis.Cancer Biol Med. 2021 Mar 12;18(2):530-46. doi: 10.20892/j.issn.2095-3941.2020.0131. Cancer Biol Med. 2021. PMID: 33710810 Free PMC article.
-
HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice.Gastroenterology. 2020 Mar;158(4):985-999.e9. doi: 10.1053/j.gastro.2019.11.031. Epub 2019 Nov 22. Gastroenterology. 2020. PMID: 31759926 Free PMC article.
-
Multiple roles and regulatory mechanisms of the transcription factor HNF4 in the intestine.Front Endocrinol (Lausanne). 2023 Aug 10;14:1232569. doi: 10.3389/fendo.2023.1232569. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635981 Free PMC article. Review.
References
-
- James R., Erler T., Kazenwadel J. (1994) Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J. Biol. Chem. 269, 15229–15237 - PubMed
-
- German M. S., Wang J., Chadwick R. B., Rutter W. J. (1992) Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 6, 2165–2176 - PubMed
-
- James R., Kazenwadel J. (1991) Homeobox gene expression in the intestinal epithelium of adult mice. J. Biol. Chem. 266, 3246–3251 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

