Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury
- PMID: 25488667
- PMCID: PMC4319034
- DOI: 10.1074/jbc.M114.603431
Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury
Abstract
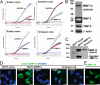
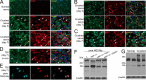
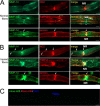
Neuronal glial antigen 2 (NG2) is an integral membrane chondroitin sulfate proteoglycan expressed by vascular pericytes, macrophages (NG2-Mφ), and progenitor glia of the nervous system. Herein, we revealed that NG2 shedding and axonal growth, either independently or jointly, depended on the pericellular remodeling events executed by membrane-type 1 matrix metalloproteinase (MT1-MMP/MMP-14). Using purified NG2 ectodomain constructs, individual MMPs, and primary NG2-Mφ cultures, we demonstrated for the first time that MMP-14 performed as an efficient and unconventional NG2 sheddase and that NG2-Mφ infiltrated into the damaged peripheral nervous system. We then characterized the spatiotemporal relationships among MMP-14, MMP-2, and tissue inhibitor of metalloproteinases-2 in sciatic nerve. Tissue inhibitor of metalloproteinases-2-free MMP-14 was observed in the primary Schwann cell cultures using the inhibitory hydroxamate warhead-based MP-3653 fluorescent reporter. In teased nerve fibers, MMP-14 translocated postinjury toward the nodes of Ranvier and its substrates, laminin and NG2. Inhibition of MMP-14 activity using the selective, function-blocking DX2400 human monoclonal antibody increased the levels of regeneration-associated factors, including laminin, growth-associated protein 43, and cAMP-dependent transcription factor 3, thereby promoting sensory axon regeneration after nerve crush. Concomitantly, DX2400 therapy attenuated mechanical hypersensitivity associated with nerve crush in rats. Together, our findings describe a new model in which MMP-14 proteolysis regulates the extracellular milieu and presents a novel therapeutic target in the damaged peripheral nervous system and neuropathic pain.
Keywords: Extracellular Matrix; MT1-MMP; Macrophage; Matrix Metalloproteinase (MMP); NG2; Nerve; Pain; Proteoglycan; Regeneration; Schwann Cells.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Chen Z. L., Yu W. M., Strickland S. (2007) Peripheral regeneration. Annu. Rev. Neurosci. 30, 209–233 - PubMed
-
- Busch S. A., Silver J. (2007) The role of extracellular matrix in CNS regeneration. Curr. Opin. Neurobiol. 17, 120–127 - PubMed
-
- Fawcett J. W. (2006) Overcoming inhibition in the damaged spinal cord. J. Neurotrauma 23, 371–383 - PubMed
-
- Nishiyama A., Komitova M., Suzuki R., Zhu X. (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10, 9–22 - PubMed
-
- Morgenstern D. A., Asher R. A., Naidu M., Carlstedt T., Levine J. M., Fawcett J. W. (2003) Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol. Cell. Neurosci. 24, 787–802 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

