The short stature homeobox 2 (Shox2)-bone morphogenetic protein (BMP) pathway regulates dorsal mesenchymal protrusion development and its temporary function as a pacemaker during cardiogenesis
- PMID: 25488669
- PMCID: PMC4303656
- DOI: 10.1074/jbc.M114.619007
The short stature homeobox 2 (Shox2)-bone morphogenetic protein (BMP) pathway regulates dorsal mesenchymal protrusion development and its temporary function as a pacemaker during cardiogenesis
Abstract
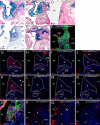
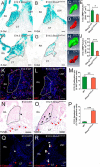
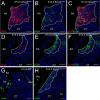
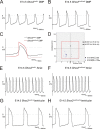
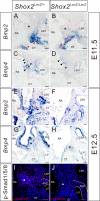
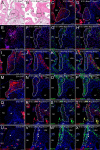
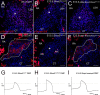
The atrioventricular (AV) junction plays a critical role in chamber septation and transmission of cardiac conduction pulses. It consists of structures that develop from embryonic dorsal mesenchymal protrusion (DMP) and the embryonic AV canal. Despite extensive studies on AV junction development, the genetic regulation of DMP development remains poorly understood. In this study we present evidence that Shox2 is expressed in the developing DMP. Intriguingly, this Shox2-expressing domain possesses a pacemaker-specific genetic profile including Hcn4 and Tbx3. This genetic profile leads to nodal-like electrophysiological properties, which is gradually silenced as the AV node becomes matured. Phenotypic analyses of Shox2(-/-) mice revealed a hypoplastic and defectively differentiated DMP, likely attributed to increased apoptosis, accompanied by dramatically reduced expression of Bmp4 and Hcn4, ectopic activation of Cx40, and an aberrant pattern of action potentials. Interestingly, conditional deletion of Bmp4 or inhibition of BMP signaling by overexpression of Noggin using a Shox2-Cre allele led to a similar DMP hypoplasia and down-regulation of Hcn4, whereas activation of a transgenic Bmp4 allele in Shox2(-/-) background attenuated DMP defects. Moreover, the lack of Hcn4 expression in the DMP of mice carrying Smad4 conditional deletion and direct binding of pSmad1/5/8 to the Hcn4 regulatory region further confirm the Shox2-BMP genetic cascade in the regulation of DMP development. Our results reveal that Shox2 regulates DMP fate and development by controlling BMP signaling through the Smad-dependent pathway to drive tissue growth and to induce Hcn4 expression and suggest a temporal pacemaking function for the DMP during early cardiogenesis.
Keywords: Bone Morphogenetic Protein (BMP); Dorsal Mesenchymal Protrusion; Gene Regulation; Heart Development; SMAD Transcription Factor; Shox2; Signal Transduction; Smad4-dependent.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Comment in
-
Does the dorsal mesenchymal protrusion act as a temporary pacemaker during heart development?J Biol Chem. 2015 Mar 20;290(12):8013-4. doi: 10.1074/jbc.L115.642207. J Biol Chem. 2015. PMID: 25795731 Free PMC article. No abstract available.
-
Reply to Kelder et al.: Does the dorsal mesenchymal protrusion act as a temporary pacemaker during heart development?J Biol Chem. 2015 Mar 20;290(12):8015. doi: 10.1074/jbc.l115.643981. J Biol Chem. 2015. PMID: 25964955 Free PMC article. No abstract available.
Similar articles
-
Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation.Circ Res. 2013 May 24;112(11):1420-32. doi: 10.1161/CIRCRESAHA.112.300821. Epub 2013 Apr 12. Circ Res. 2013. PMID: 23584254 Free PMC article.
-
Shox2 regulates the pacemaker gene program in embryoid bodies.Stem Cells Dev. 2013 Nov 1;22(21):2915-26. doi: 10.1089/scd.2013.0123. Epub 2013 Jul 26. Stem Cells Dev. 2013. PMID: 23767866
-
A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node.Development. 2015 Jul 15;142(14):2521-32. doi: 10.1242/dev.120220. Epub 2015 Jul 2. Development. 2015. PMID: 26138475 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Shox2: The Role in Differentiation and Development of Cardiac Conduction System.Tohoku J Exp Med. 2018 Mar;244(3):177-186. doi: 10.1620/tjem.244.177. Tohoku J Exp Med. 2018. PMID: 29503396 Review.
Cited by
-
Bone morphogenetic protein 4 promotes the differentiation of Tbx18-positive epicardial progenitor cells to pacemaker-like cells.Exp Ther Med. 2019 Apr;17(4):2648-2656. doi: 10.3892/etm.2019.7243. Epub 2019 Feb 4. Exp Ther Med. 2019. PMID: 30906456 Free PMC article.
-
Evaluation of the Potential Targets of Shenxian-Shengmai Oral Liquid in Treating Sick Sinus Syndrome Based on Network Pharmacology and Molecular Docking.Food Sci Nutr. 2024 Nov 12;12(12):10517-10534. doi: 10.1002/fsn3.4587. eCollection 2024 Dec. Food Sci Nutr. 2024. PMID: 39723092 Free PMC article.
-
The method of sinus node-like pacemaker cells from human induced pluripotent stem cells by BMP and Wnt signaling.Cell Biol Toxicol. 2023 Dec;39(6):2725-2741. doi: 10.1007/s10565-023-09797-7. Epub 2023 Mar 1. Cell Biol Toxicol. 2023. PMID: 36856942
-
The Transcription Factor Shox2 Shapes Neuron Firing Properties and Suppresses Seizures by Regulation of Key Ion Channels in Thalamocortical Neurons.Cereb Cortex. 2021 Jun 10;31(7):3194-3212. doi: 10.1093/cercor/bhaa414. Cereb Cortex. 2021. PMID: 33675359 Free PMC article.
-
Hox-dependent coordination of mouse cardiac progenitor cell patterning and differentiation.Elife. 2020 Aug 17;9:e55124. doi: 10.7554/eLife.55124. Elife. 2020. PMID: 32804075 Free PMC article.
References
-
- Mangoni M. E., Nargeot J. (2008) Genesis and regulation of the heart automaticity. Physiol. Rev. 88, 919–982 - PubMed
-
- Wessels A., Markman M. W., Vermeulen J. L., Anderson R. H., Moorman A. F., Lamers W. H. (1996) The development of the atrioventricular junction in the human heart. Circ. Res. 78, 110–117 - PubMed
-
- Christoffels V. M., Smits G. J., Kispert A., Moorman A. F. (2010) Development of the pacemaker tissues of the heart. Circ. Res. 106, 240–254 - PubMed
-
- Briggs L. E., Phelps A. L., Brown E., Kakarla J., Anderson R. H., van den Hoff M. J., Wessels A. (2013) Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ. Res. 112, 1420–1432 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

