Exosomes and other extracellular vesicles in host-pathogen interactions
- PMID: 25488940
- PMCID: PMC4304727
- DOI: 10.15252/embr.201439363
Exosomes and other extracellular vesicles in host-pathogen interactions
Abstract
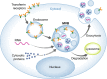
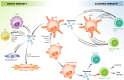
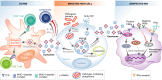
An effective immune response requires the engagement of host receptors by pathogen-derived molecules and the stimulation of an appropriate cellular response. Therefore, a crucial factor in our ability to control an infection is the accessibility of our immune cells to the foreign material. Exosomes-which are extracellular vesicles that function in intercellular communication-may play a key role in the dissemination of pathogen- as well as host-derived molecules during infection. In this review, we highlight the composition and function of exosomes and other extracellular vesicles produced during viral, parasitic, fungal and bacterial infections and describe how these vesicles could function to either promote or inhibit host immunity.
Keywords: exosomes; extracellular vesicles; immunity; pathogens.
© 2014 The Authors.
Figures

References
-
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. - PubMed
-
- Rana RR, Simpson P, Zhang M, Jennions M, Ukegbu C, Spear AM, Alguel Y, Matthews SJ, Atkins HS, Byrne B. Yersinia pestis TIR-domain protein forms dimers that interact with the human adaptor protein MyD88. Microb Pathog. 2011;51:89–95. - PubMed
-
- Yadav M, Roach SK, Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 2004;172:5588–5597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

