Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial
- PMID: 25488963
- PMCID: PMC4279237
- DOI: 10.1200/JCO.2013.54.3298
Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial
Erratum in
-
Errata.J Clin Oncol. 2017 Aug 1;35(22):2590. doi: 10.1200/JCO.2017.74.5729. J Clin Oncol. 2017. PMID: 28750184 Free PMC article. No abstract available.
Abstract
Purpose: This open-label phase III trial evaluated efficacy and tolerability of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy.
Patients and methods: Patients were randomly assigned in a 1:1 ratio to linifanib 17.5 mg once daily or sorafenib 400 mg twice daily. Patients were stratified by region (Outside Asia, Japan, and rest of Asia), Eastern Cooperative Oncology Group performance score (ECOG PS; 0 or 1), vascular invasion or extrahepatic spread (yes or no), and hepatitis B virus (HBV) infection (yes or no). The primary end point of the study was overall survival (OS). Secondary end points were time to progression (TTP) and objective response rate (ORR) per RECIST v1.1.
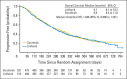
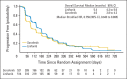
Results: We randomly assigned 1,035 patients (median age, 60 years; Asian, 66.6%; ECOG PS 0, 65.2%; HBV, 49.1%; vascular invasion or extrahepatic spread, 70.1%). Median OS was 9.1 months on the linifanib arm (95% CI, 8.1 to 10.2) and 9.8 months on the sorafenib arm (95% CI, 8.3 to 11.0; hazard ratio [HR], 1.046; 95% CI, 0.896 to 1.221). For prespecified stratification subgroups, OS HRs ranged from 0.793 to 1.119 and the 95% CI contained 1.0. Median TTP was 5.4 months on the linifanib arm (95% CI, 4.2 to 5.6) and 4.0 months on the sorafenib arm (95% CI, 2.8 to 4.2; HR, 0.759; 95% CI, 0.643 to 0.895; P = .001). Best response rate was 13.0% on the linifanib arm versus 6.9% on the sorafenib arm. Grade 3/4 adverse events (AEs); serious AEs; and AEs leading to discontinuation, dose interruption, and reduction were more frequent with linifanib (all P < .001).
Conclusion: Linifanib and sorafenib had similar OS in advanced HCC. Predefined superiority and noninferiority OS boundaries were not met for linifanib and the study failed to meet the primary end point. TTP and ORR favored linifanib; safety results favored sorafenib.
Trial registration: ClinicalTrials.gov NCT01009593.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



Comment in
-
Reply to M. Bouattour et al.J Clin Oncol. 2015 Aug 1;33(22):2486. doi: 10.1200/JCO.2015.61.6730. Epub 2015 Jun 1. J Clin Oncol. 2015. PMID: 26033815 No abstract available.
-
Negative Trials for Foreseeable Safety Reasons in Advanced Hepatocellular Carcinoma: How Long Are We Going to Take Lightly Pharmacokinetics of Tyrosine Kinase Inhibitors?J Clin Oncol. 2015 Aug 1;33(22):2484-5. doi: 10.1200/JCO.2014.60.6954. Epub 2015 Jun 1. J Clin Oncol. 2015. PMID: 26033820 No abstract available.
References
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- World Health Organization. Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/
-
- Kirk GD, Lesi OA, Mendy M, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211–219. - PubMed
-
- McGlynn KA, Tsao L, Hsing AW, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–296. - PubMed
-
- American Cancer Society. Global Cancer Facts and Figures 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

