Protein phosphatase 2A holoenzyme is targeted to peroxisomes by piggybacking and positively affects peroxisomal β-oxidation
- PMID: 25489022
- PMCID: PMC4326747
- DOI: 10.1104/pp.114.254409
Protein phosphatase 2A holoenzyme is targeted to peroxisomes by piggybacking and positively affects peroxisomal β-oxidation
Abstract
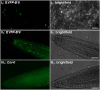
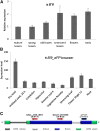
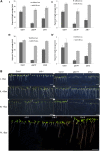
The eukaryotic, highly conserved serine (Ser)/threonine-specific protein phosphatase 2A (PP2A) functions as a heterotrimeric complex composed of a catalytic (C), scaffolding (A), and regulatory (B) subunit. In Arabidopsis (Arabidopsis thaliana), five, three, and 17 genes encode different C, A, and B subunits, respectively. We previously found that a B subunit, B'θ, localized to peroxisomes due to its C-terminal targeting signal Ser-Ser-leucine. This work shows that PP2A C2, C5, andA2 subunits interact and colocalize with B'θ in peroxisomes. C and A subunits lack peroxisomal targeting signals, and their peroxisomal import depends on B'θ and appears to occur by piggybacking transport. B'θ knockout mutants were impaired in peroxisomal β-oxidation as shown by developmental arrest of seedlings germinated without sucrose, accumulation of eicosenoic acid, and resistance to protoauxins indole-butyric acid and 2,4-dichlorophenoxybutyric acid. All of these observations strongly substantiate that a full PP2A complex is present in peroxisomes and positively affects β-oxidation of fatty acids and protoauxins.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



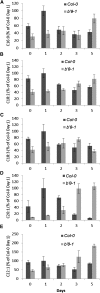
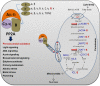
References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 - PubMed
-
- Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152: 141–145 - PubMed
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
-
- Coca M, San Segundo B (2010) AtCPK1 calcium-dependent protein kinase mediates pathogen resistance in Arabidopsis. Plant J 63: 526–540 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

