Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase
- PMID: 25489112
- PMCID: PMC4280606
- DOI: 10.1073/pnas.1419701112
Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase
Abstract
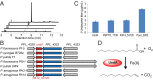
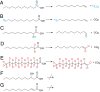
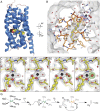
Aliphatic medium-chain 1-alkenes (MCAEs, ∼10 carbons) are "drop-in" compatible next-generation fuels and precursors to commodity chemicals. Mass production of MCAEs from renewable resources holds promise for mitigating dependence on fossil hydrocarbons. An MCAE, such as 1-undecene, is naturally produced by Pseudomonas as a semivolatile metabolite through an unknown biosynthetic pathway. We describe here the discovery of a single gene conserved in Pseudomonas responsible for 1-undecene biosynthesis. The encoded enzyme is able to convert medium-chain fatty acids (C10-C14) into their corresponding terminal olefins using an oxygen-activating, nonheme iron-dependent mechanism. Both biochemical and X-ray crystal structural analyses suggest an unusual mechanism of β-hydrogen abstraction during fatty acid substrate activation. Our discovery unveils previously unidentified chemistry in the nonheme Fe(II) enzyme family, provides an opportunity to explore the biology of 1-undecene in Pseudomonas, and paves the way for tailored bioconversion of renewable raw materials to MCAE-based biofuels and chemical commodities.
Keywords: biofuel; biosynthesis; hydrocarbon; iron-dependent desaturase/decarboxylase; terminal olefin.
Conflict of interest statement
Conflict of interest statement: Z.R. and W.Z. have filed a provisional patent application on aspects of this research.
Figures
References
-
- Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488(7411):320–328. - PubMed
-
- Ray S, Rao PVC, Choudary NV. Poly-alpha-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubr Sci. 2012;24(1):23–44.
-
- Moiseenkov AM, Schaub B, Margot C, Schlosser M. A new stereoselective synthesis of (Z)-9-tricosene, the sex attractant of the common housefly. Tetrahedron Lett. 1985;26(3):305–306.
-
- Kai M, et al. Bacterial volatiles and their action potential. Appl Microbiol Biotechnol. 2009;81(6):1001–1012. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

