Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization
- PMID: 25489223
- PMCID: PMC4225142
Lack of dystrophin protein Dp71 results in progressive cataract formation due to loss of fiber cell organization
Abstract
Purpose: Dp71 is the main product of the Duchenne muscular dystrophy (DMD) gene in the central nervous system. While studying the impact of its absence on retinal functions, we discovered that mice lacking Dp71 also developed a progressive opacification of the crystalline lens. The purpose of this study was to perform a detailed characterization of the cataract formation in Dp71 knockout (KO-Dp71) mice.
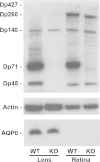
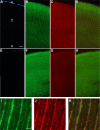
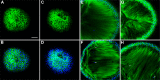
Methods: Cataract formations in KO-Dp71 mice and wild-type (wt) littermates were assessed in vivo by slit-lamp examination and ex vivo by histological analysis as a function of aging. The expression and cellular localization of the DMD gene products were monitored by western blot and immunohistochemical analysis. Fiber cell integrity was assessed by analyzing the actin cytoskeleton as well as the expression of aquaporin-0 (AQP0).
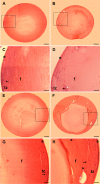
Results: As expected, a slit-lamp examination revealed that only one of the 20 tested wt animals presented with a mild opacification of the lens and only at the most advanced age. However, a lack of Dp71 was associated with a 40% incidence of cataracts as early as 2 months of age, which progressively increased to full penetrance by 7 months. A subsequent histological analysis revealed an alteration in the structures of the lenses of KO-Dp71 mice that correlated with the severity of the lens opacity. An analysis of the expression of the different dystrophin gene products revealed that Dp71 was the major DMD gene product expressed in the lens, especially in fiber cells. The role of Dp71 in fiber cells was also suggested by the progressive disorganization of the lens fibers, which was observed in the absence of Dp71 and demonstrated by irregular staining of the actin network and the aqueous channel AQP0.
Conclusions: While its role in the retina has been well characterized, this study demonstrates for the first time the role played by Dp71 in a different ocular tissue: the crystalline lens. It primarily demonstrates the role that Dp71 plays in the maintenance of the integrity of the secondary lens fibers.
Figures


References
-
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–17. - PubMed
-
- Holt KH, Campbell KP. Assembly of the sarcoglycan complex. Insights for muscular dystrophy. J Biol Chem. 1998;273:34667–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

