Fabrication, characterization, and thermal property evaluation of silver nanofluids
- PMID: 25489293
- PMCID: PMC4256963
- DOI: 10.1186/1556-276X-9-645
Fabrication, characterization, and thermal property evaluation of silver nanofluids
Abstract
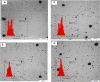
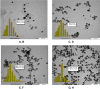
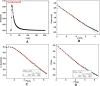
Silver nanoparticles were successfully prepared in two different solvents using a microwave heating technique, with various irradiation times. The silver nanoparticles were dispersed in polar liquids (distilled water and ethylene glycol) without any other reducing agent, in the presence of the stabilizer polyvinylpyrrolidone (PVP). The optical properties, thermal properties, and morphology of the synthesized silver particles were characterized using ultraviolet-visible spectroscopy, photopyroelectric technique, and transmission electron microscopy. It was found that for the both solvents, the effect of microwave irradiation was mainly on the particles distribution, rather than the size, which enabled to make stable and homogeneous silver nanofluids. The individual spherical nanostructure of self-assembled nanoparticles has been formed during microwave irradiation. Ethylene glycol solution, due to its special properties, such as high dielectric loss, high molecular weight, and high boiling point, can serve as a good solvent for microwave heating and is found to be a more suitable medium than the distilled water. A photopyroelectric technique was carried out to measure thermal diffusivity of the samples. The precision and accuracy of this technique was established by comparing the measured thermal diffusivity of the distilled water and ethylene glycol with values reported in the literature. The thermal diffusivity ratio of the silver nanofluids increased up to 1.15 and 1.25 for distilled water and ethylene glycol, respectively.
Keywords: Microwave heating; Nanofluids; Photopyroelectric; Silver nanoparticles; Thermal diffusivity.
Figures






References
-
- Wong KV, De Leon O. Applications of nanofluids: current and future. Adv Mechanical Eng. 2010;2010:11.
-
- Huaqing X, Hua G, Motoo F, Xing Z. Short hot wire technique for measuring thermal conductivity and thermal diffusivity of various materials. Meas Sci Technol. 2006;17:208. doi: 10.1088/0957-0233/17/1/032. - DOI
-
- Ali F, Yunus W. Study of the effect of volume fraction concentration and particle materials on thermal conductivity and thermal diffusivity of nanofluids. Jpn J Appl Phys. 2011;50:085201. doi: 10.7567/JJAP.50.085201. - DOI
-
- Zhang X, Gu H, Fujii M. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Experimental Thermal and Fluid Science. 2007;31:593–599. doi: 10.1016/j.expthermflusci.2006.06.009. - DOI
-
- Murshed S, Leong K, Yang C. Determination of the effective thermal diffusivity of nanofluids by the double hot-wire technique. J Phys D Appl Phys. 2006;39:5316. doi: 10.1088/0022-3727/39/24/033. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

