Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial
- PMID: 25489785
- PMCID: PMC5447287
- DOI: 10.1164/rccm.201410-1843OC
Daily rifapentine for treatment of pulmonary tuberculosis. A randomized, dose-ranging trial
Erratum in
-
Erratum: daily rifapentine for treatment of pulmonary tuberculosis: a randomized, dose-ranging trial.Am J Respir Crit Care Med. 2015 May 15;191(10):1210. doi: 10.1164/rccm.19110erratum2. Am J Respir Crit Care Med. 2015. PMID: 25978581 Free PMC article. No abstract available.
Abstract
Rationale: Rifapentine has potent activity in mouse models of tuberculosis chemotherapy but its optimal dose and exposure in humans are unknown.
Objectives: We conducted a randomized, partially blinded dose-ranging study to determine tolerability, safety, and antimicrobial activity of daily rifapentine for pulmonary tuberculosis treatment.
Methods: Adults with sputum smear-positive pulmonary tuberculosis were assigned rifapentine 10, 15, or 20 mg/kg or rifampin 10 mg/kg daily for 8 weeks (intensive phase), with isoniazid, pyrazinamide, and ethambutol. The primary tolerability end point was treatment discontinuation. The primary efficacy end point was negative sputum cultures at completion of intensive phase.
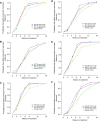
Measurements and main results: A total of 334 participants were enrolled. At completion of intensive phase, cultures on solid media were negative in 81.3% of participants in the rifampin group versus 92.5% (P = 0.097), 89.4% (P = 0.29), and 94.7% (P = 0.049) in the rifapentine 10, 15, and 20 mg/kg groups. Liquid cultures were negative in 56.3% (rifampin group) versus 74.6% (P = 0.042), 69.7% (P = 0.16), and 82.5% (P = 0.004), respectively. Compared with the rifampin group, the proportion negative at the end of intensive phase was higher among rifapentine recipients who had high rifapentine areas under the concentration-time curve. Percentages of participants discontinuing assigned treatment for reasons other than microbiologic ineligibility were similar across groups (rifampin, 8.2%; rifapentine 10, 15, or 20 mg/kg, 3.4, 2.5, and 7.4%, respectively).
Conclusions: Daily rifapentine was well-tolerated and safe. High rifapentine exposures were associated with high levels of sputum sterilization at completion of intensive phase. Further studies are warranted to determine if regimens that deliver high rifapentine exposures can shorten treatment duration to less than 6 months. Clinical trial registered with www.clinicaltrials.gov (NCT 00694629).
Trial registration: ClinicalTrials.gov NCT00694629.
Keywords: mycobacterium; rifamycins; rifapentine; therapeutics; tuberculosis.
Figures
Comment in
-
Optimizing the dose of rifapentine to treat tuberculosis. It's elementary.Am J Respir Crit Care Med. 2015 Feb 1;191(3):251-2. doi: 10.1164/rccm.201412-2257ED. Am J Respir Crit Care Med. 2015. PMID: 25635488 No abstract available.
References
-
- Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med. 1980;1:231–241. - PubMed
-
- Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4:796–806. - PubMed
-
- Ji B, Truffot-Pernot C, Lacroix C, Raviglione MC, O’Brien RJ, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. - PubMed

