Monitoring of transplanted liver health by quantification of organ-specific genomic marker in circulating DNA from receptor
- PMID: 25489845
- PMCID: PMC4260920
- DOI: 10.1371/journal.pone.0113987
Monitoring of transplanted liver health by quantification of organ-specific genomic marker in circulating DNA from receptor
Abstract
Background: Health assessment of the transplanted organ is very important due to the relationship of long-term survival of organ transplant recipients and health organ maintenance. Nowadays, the measurement of cell-free DNA from grafts in the circulation of transplant recipients has been considered a potential biomarker of organ rejection or transplant associated complications in an attempt to replace or reduce liver biopsy. However, methods developed to date are expensive and extremely time-consuming. Our approach was to measure the SRY gene, as a male organ biomarker, in a setting of sex-mismatched female recipients of male donor organs.
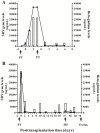
Methods: Cell-free DNA quantization of the SRY gene was performed by real-time quantitative PCR beforehand, at the moment of transplantation during reperfusion (day 0) and during the stay at the intensive care unit. Beta-globin cell-free DNA levels, a general cellular damage marker, were also quantified.
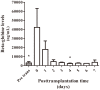
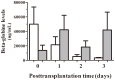
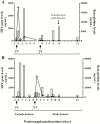
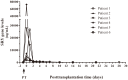
Results: Beta-globin mean values of patients, who accepted the graft without any complications during the first week after surgery, diminished from day 0 until patient stabilization. This decrease was not so evident in patients who suffered some kind of post-transplantation complications. All patients showed an increase in SRY levels at day 0, which decreased during hospitalization. Different complications that did not compromise donated organs showed increased beta-globin levels but no SRY gene levels. However, when a donated organ was damaged the patients exhibited high levels of both genes.
Conclusion: Determination of a SRY gene in a female recipient's serum is a clear and specific biomarker of donated organs and may give us important information about graft health in a short period of time by a non-expensive technique. This approach may permit clinicians to maintain a close follow up of the transplanted patient.
Conflict of interest statement
Figures
References
-
- Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, et al. (2013) The evolutions of liver transplantations during 3 decades, analysis of 5347 consecutive liver transplants at a single center. Ann Surg 258:409–21. - PubMed
-
- Millán O, Rafael-Valdivia L, Torrademé E, López A, Fortuna V, et al. (2013) Intracellular IFN-γ and IL-2 expression monitoring as surrogate markers of the risk of acute rejection and personal drug response in de novo liver transplant recipients. Cytokine 61(2):556–64. - PubMed
-
- Vivarelli M, Smith HM, Naoumov NV, Williams R (1995) Quantitative assessment of serum beta-2-microglobulin in liver transplant recipients and relationship to liver graft rejection. Eur J Gastroenterol Hepatol 7(12):1215–9. - PubMed
-
- Macher H, Egea-Guerrero JJ, Revuelto-Rey J, Gordillo-Escobar E, Enamorado-Enamorado J, et al. (2012) Role of early cell-free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clin Chim Acta 24(414):12–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

