Marine origin collagens and its potential applications
- PMID: 25490254
- PMCID: PMC4278207
- DOI: 10.3390/md12125881
Marine origin collagens and its potential applications
Abstract
Collagens are the most abundant high molecular weight proteins in both invertebrate and vertebrate organisms, including mammals, and possess mainly a structural role, existing different types according with their specific organization in distinct tissues. From this, they have been elected as one of the key biological materials in tissue regeneration approaches. Also, industry is constantly searching for new natural sources of collagen and upgraded methodologies for their production. The most common sources are from bovine and porcine origin, but other ways are making their route, such as recombinant production, but also extraction from marine organisms like fish. Different organisms have been proposed and explored for collagen extraction, allowing the sustainable production of different types of collagens, with properties depending on the kind of organism (and their natural environment) and extraction methodology. Such variety of collagen properties has been further investigated in different ways to render a wide range of applications. The present review aims to shed some light on the contribution of marine collagens for the scientific and technological development of this sector, stressing the opportunities and challenges that they are and most probably will be facing to assume a role as an alternative source for industrial exploitation.
Figures

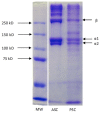

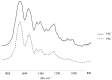
References
-
- Vanderrest M., Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

