Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development
- PMID: 25490431
- PMCID: PMC6270949
- DOI: 10.3390/molecules191220391
Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development
Abstract
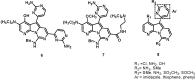
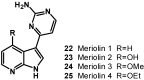
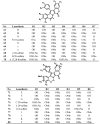
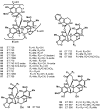
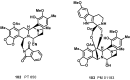
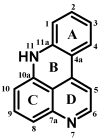
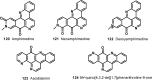
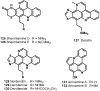
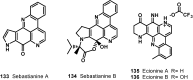
The present review describes research on novel natural antitumor alkaloids isolated from marine invertebrates. The structure, origin, and confirmed cytotoxic activity of more than 130 novel alkaloids belonging to several structural families (indoles, pyrroles, pyrazines, quinolines, and pyridoacridines), together with some of their synthetic analogs, are illustrated. Recent discoveries concerning the current state of the potential and/or development of some of them as new drugs, as well as the current knowledge regarding their modes of action, are also summarized. A special emphasis is given to the role of marine invertebrate alkaloids as an important source of leads for anticancer drug discovery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

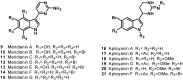
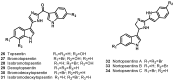
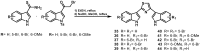
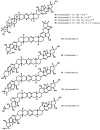
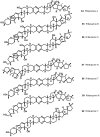
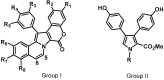



References
-
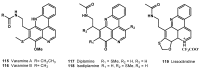
- Perry N.B., Ettoouati L., Litaudon M., Blunt J.W., Munro M.H.G., Parkin S., Hope H. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Part 1: Variolin B, a new antitumor and antiviral compound. Tetrahedron. 1994;50:3987–3992. doi: 10.1016/S0040-4020(01)89673-3. - DOI
-
- Trimurtulu G., Faulkner D.J., Perry N.B., Ettouati L., Litaudon M., Blunt J.W., Munro M.H.G., Jameson G.B. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Part 2: Variolin A and N(3')-methyl tetrahydrovariolin B. Tetrahedron. 1994;50:3993–4000. doi: 10.1016/S0040-4020(01)89674-5. - DOI
-
- Anderson R.J., Morris J.C. Total synthesis of variolin B. Tetrahedron Lett. 2001;42:8697–8699. doi: 10.1016/S0040-4039(01)01881-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

