Slit molecules prevent entrance of trunk neural crest cells in developing gut
- PMID: 25490618
- PMCID: PMC4355086
- DOI: 10.1016/j.ijdevneu.2014.12.003
Slit molecules prevent entrance of trunk neural crest cells in developing gut
Abstract
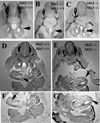
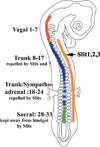
Neural crest cells emerge from the dorsal neural tube early in development and give rise to sensory and sympathetic ganglia, adrenal cells, teeth, melanocytes and especially enteric nervous system. Several inhibitory molecules have been shown to play important roles in neural crest migration, among them are the chemorepulsive Slit1-3. It was known that Slits chemorepellants are expressed at the entry to the gut, and thus could play a role in the differential ability of vagal but not trunk neural crest cells to invade the gut and form enteric ganglia. Especially since trunk neural crest cells express Robo receptor while vagal do not. Thus, although we know that Robo mediates migration along the dorsal pathway in neural crest cells, we do not know if it is responsible in preventing their entry into the gut. The goal of this study was to further corroborate a role for Slit molecules in keeping trunk neural crest cells away from the gut. We observed that when we silenced Robo receptor in trunk neural crest, the sympathoadrenal (somites 18-24) were capable of invading gut mesenchyme in larger proportion than more rostral counterparts. The more rostral trunk neural crest tended not to migrate beyond the ventral aorta, suggesting that there are other repulsive molecules keeping them away from the gut. Interestingly, we also found that when we silenced Robo in sacral neural crest they did not wait for the arrival of vagal crest but entered the gut and migrated rostrally, suggesting that Slit molecules are the ones responsible for keeping them waiting at the hindgut mesenchyme. These combined results confirm that Slit molecules are responsible for keeping the timeliness of colonization of the gut by neural crest cells.
Keywords: Cell migration; Enteric nervous system; Neural crest; Robo.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia.Dev Biol. 2000 Mar 1;219(1):30-43. doi: 10.1006/dbio.1999.9592. Dev Biol. 2000. PMID: 10677253
-
Effects of different regions of the developing gut on the migration of enteric neural crest-derived cells: a role for Sema3A, but not Sema3F.Dev Biol. 2007 May 1;305(1):287-99. doi: 10.1016/j.ydbio.2007.02.020. Epub 2007 Feb 21. Dev Biol. 2007. PMID: 17362911
-
Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells.J Cell Biol. 2003 Jul 21;162(2):269-79. doi: 10.1083/jcb.200301041. J Cell Biol. 2003. PMID: 12876276 Free PMC article.
-
Guidance cues involved in the development of the peripheral autonomic nervous system.Auton Neurosci. 2004 May 31;112(1-2):1-14. doi: 10.1016/j.autneu.2004.02.008. Auton Neurosci. 2004. PMID: 15233925 Review.
-
Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:3-7. doi: 10.1111/j.1743-3150.2004.00466.x. Neurogastroenterol Motil. 2004. PMID: 15065996 Review.
Cited by
-
Large intestine embryogenesis: Molecular pathways and related disorders (Review).Int J Mol Med. 2020 Jul;46(1):27-57. doi: 10.3892/ijmm.2020.4583. Epub 2020 Apr 21. Int J Mol Med. 2020. PMID: 32319546 Free PMC article. Review.
-
Origins of Aortic Coarctation: A Vascular Smooth Muscle Compartment Boundary Model.J Dev Biol. 2025 Apr 18;13(2):13. doi: 10.3390/jdb13020013. J Dev Biol. 2025. PMID: 40265371 Free PMC article.
-
Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5570-5575. doi: 10.1073/pnas.1814930116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819894 Free PMC article.
-
Enteric nervous system development: A crest cell's journey from neural tube to colon.Semin Cell Dev Biol. 2017 Jun;66:94-106. doi: 10.1016/j.semcdb.2017.01.006. Epub 2017 Jan 10. Semin Cell Dev Biol. 2017. PMID: 28087321 Free PMC article. Review.
-
Screen for Slit/Robo signaling in trunk neural cells reveals new players.Gene Expr Patterns. 2018 Jun;28:22-33. doi: 10.1016/j.gep.2018.01.002. Epub 2018 Feb 7. Gene Expr Patterns. 2018. PMID: 29427758 Free PMC article.
References
-
- Allan IJ, Newgreen DF. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157:137–154. - PubMed
-
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. - PubMed
-
- Bronner-Fraser M, Stern CD, Fraser S. Analysis of neural crest cell lineage and migration. J Craniofac Genet Dev Biol. 1991;11:214–222. - PubMed
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. - PubMed
-
- Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

