Stereoconvergent [1,2]- and [1,4]-Wittig rearrangements of 2-silyl-6-aryl-5,6-dihydropyrans: a tale of steric vs electronic regiocontrol of divergent pathways
- PMID: 25490725
- PMCID: PMC4301091
- DOI: 10.1021/jo5026942
Stereoconvergent [1,2]- and [1,4]-Wittig rearrangements of 2-silyl-6-aryl-5,6-dihydropyrans: a tale of steric vs electronic regiocontrol of divergent pathways
Abstract
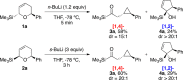
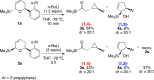
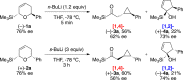
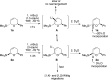
The regiodivergent ring contraction of diastereomeric 2-silyl-5,6-dihydro-6-aryl-(2H)-pyrans via [1,2]- and [1,4]-Wittig rearrangements to the corresponding α-silylcyclopentenols or (α-cyclopropyl)acylsilanes favor the [1,4]-pathway by ortho and para directing groups in the aromatic appendage and/or by sterically demanding silyl groups. The [1,2]-pathway is dominant with meta directing or electron-poor aromatic moieties. Exclusive [1,2]-Wittig rearrangements are observed when olefin substituents proximal to the silyl are present. cis and trans diastereomers exhibit different reactivities, but converge to a single [1,2]- or [1,4]-Wittig product with high diastereoselectivity and yield.
Figures







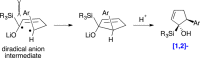








References
-
- Wittig G.; Lohmann L. Justus Liebigs Ann. Chem. 1942, 550, 260–268.
-
- Nakai T.; Mikami K. Chem. Rev. 1986, 86, 885–902.
-
- Nakai T.; Tomooka K. Pure Appl. Chem. 1997, 69, 595–600.
-
- Tomooka K.; Yamamoto H.; Nakai T. Liebigs Ann./Recl. 1997, 1275–1281.
-
- Felkin H.; Tambute A. Tetrahedron Lett. 1969, 821–822.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

