Nitrogenase and homologs
- PMID: 25491285
- PMCID: PMC4336585
- DOI: 10.1007/s00775-014-1225-3
Nitrogenase and homologs
Abstract
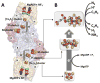
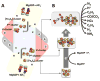
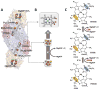
Nitrogenase catalyzes biological nitrogen fixation, a key step in the global nitrogen cycle. Three homologous nitrogenases have been identified to date, along with several structural and/or functional homologs of this enzyme that are involved in nitrogenase assembly, bacteriochlorophyll biosynthesis and methanogenic process, respectively. In this article, we provide an overview of the structures and functions of nitrogenase and its homologs, which highlights the similarity and disparity of this uniquely versatile group of enzymes.
Figures
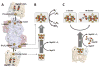
References
-
- Burgess BK, Lowe DJ. Mechanism of molybdenum nitrogenase. Chem Rev. 1996;96:2983–3012. - PubMed
-
- Eady RR. Structure-function relationships of alternative nitrogenases. Chem Rev. 1996;96:3013–3030. - PubMed
-
- Rees DC, Akif Tezcan F, Haynes CA, Walton MY, Andrade S, Einsle O, Howard JB. Structural basis of biological nitrogen fixation. Philos Trans A Math Phys Eng Sci. 2005;363:971–984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

