KRAS mutations in tumor tissue and plasma by different assays predict survival of patients with metastatic colorectal cancer
- PMID: 25491325
- PMCID: PMC4272803
- DOI: 10.1186/s13046-014-0104-7
KRAS mutations in tumor tissue and plasma by different assays predict survival of patients with metastatic colorectal cancer
Abstract
Background: The optimal laboratory assay for detecting KRAS mutations in different biospecimens from patients with metastatic colorectal cancer (mCRC), and the clinical relevance of these gene alterations is still in question. We analyzed the prognostic-predictive relevance of KRAS status, determined in tumor and plasma DNA by two different assays, in a large mono-institutional series of mCRC patients.
Methods: DNA sequencing and peptide-nucleic-acid-mediated-polymerase chain reaction clamping (PNA-PCR) were used to determine KRAS status in 416 tumor and 242 matched plasma DNA samples from mCRC patients who received chemotherapy only. Relationships with outcomes were analyzed with respect to the different assays and tissue types.
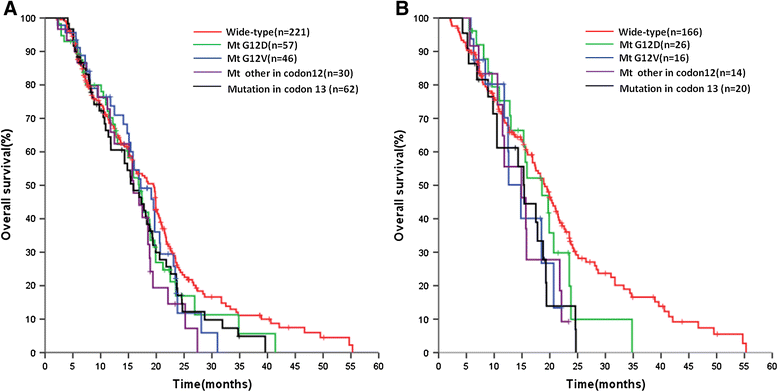
Results: PNA-PCR was significantly more sensitive in detecting KRAS mutations than sequencing (41% vs. 30%, p < 0.001). KRAS mutations were more frequent in tumor tissue than in plasma (sequencing, 38% vs. 17%, p < 0.001; PNA-PCR, 47% vs. 31%, p < 0.001). Median OS was consistently shorter in KRAS-mutated patients than KRAS wild-type patients, independent from the assay and tissue tested; the largest difference was in plasma samples analyzed by PNA-PCR (KRAS mutated vs. wild-type: 15.7 vs. 19.1 months, p = 0.009). No association was observed between KRAS status and other outcomes. When tumor and plasma results were considered together, median OS in patients categorized as tissue/plasma KRAS negative/negative, tissue/plasma KRAS discordant, and tissue/plasma KRAS positive/positive were 21.0, 16.9 and 15.4 months, respectively (p = 0.008).
Conclusions: KRAS mutation status is of prognostic relevance in patients with mCRC. KRAS mutations in both tumor tissue and plasma are a strong prognostic marker for poor outcomes.
Figures

Similar articles
-
Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial.Lancet Oncol. 2015 Aug;16(8):937-48. doi: 10.1016/S1470-2045(15)00138-2. Epub 2015 Jul 13. Lancet Oncol. 2015. PMID: 26184520 Free PMC article.
-
Changes in mutational status during third-line treatment for metastatic colorectal cancer--results of consecutive measurement of cell free DNA, KRAS and BRAF in the plasma.Int J Cancer. 2014 Nov 1;135(9):2215-22. doi: 10.1002/ijc.28863. Epub 2014 Apr 17. Int J Cancer. 2014. PMID: 24659028
-
Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer.Int J Cancer. 2015 Jan 1;136(1):83-90. doi: 10.1002/ijc.28955. Epub 2014 May 28. Int J Cancer. 2015. PMID: 24806288
-
KRAS status and resistance to epidermal growth factor receptor tyrosine-kinase inhibitor treatment in patients with metastatic colorectal cancer: a meta-analysis.Colorectal Dis. 2014 Nov;16(11):O370-8. doi: 10.1111/codi.12749. Colorectal Dis. 2014. PMID: 25155261 Review.
-
KRAS mutation testing in metastatic colorectal cancer.World J Gastroenterol. 2012 Oct 7;18(37):5171-80. doi: 10.3748/wjg.v18.i37.5171. World J Gastroenterol. 2012. PMID: 23066310 Free PMC article. Review.
Cited by
-
KRAS testing of patients with metastatic colorectal cancer in a community-based oncology setting: a retrospective database analysis.J Exp Clin Cancer Res. 2015 Mar 27;34(1):29. doi: 10.1186/s13046-015-0146-5. J Exp Clin Cancer Res. 2015. PMID: 25888436 Free PMC article.
-
Elevated kinesin family member 26B is a prognostic biomarker and a potential therapeutic target for colorectal cancer.J Exp Clin Cancer Res. 2015 Feb 5;34(1):13. doi: 10.1186/s13046-015-0129-6. J Exp Clin Cancer Res. 2015. PMID: 25652119 Free PMC article.
-
Identification of novel candidate drivers connecting different dysfunctional levels for lung adenocarcinoma using protein-protein interactions and a shortest path approach.Sci Rep. 2016 Jul 14;6:29849. doi: 10.1038/srep29849. Sci Rep. 2016. PMID: 27412431 Free PMC article.
-
Circulating Cell-Free Tumour DNA in the Management of Cancer.Int J Mol Sci. 2015 Jun 19;16(6):14122-42. doi: 10.3390/ijms160614122. Int J Mol Sci. 2015. PMID: 26101870 Free PMC article. Review.
-
KRAS Mutations in Cholangiocarcinoma: Prevalence, Prognostic Value, and KRAS G12/G13 Detection in Cell-Free DNA.Cancer Genomics Proteomics. 2025 Jan-Feb;22(1):112-126. doi: 10.21873/cgp.20492. Cancer Genomics Proteomics. 2025. PMID: 39730186 Free PMC article.
References
-
- Santini D, Loupakis F, Vincenzi B, Floriani I, Stasi I, Canestrari E, Rulli E, Maltese PE, Andreoni F, Masi G, Graziano F, Bald GG, Salvatore L, Russo A, Perrone G, Tommasino MR, Magnani M, Falcone A, Tonini G, Ruzzo A. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270–1275. doi: 10.1634/theoncologist.2008-0181. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

