Expanding the phenotype of PRPS1 syndromes in females: neuropathy, hearing loss and retinopathy
- PMID: 25491489
- PMCID: PMC4272780
- DOI: 10.1186/s13023-014-0190-9
Expanding the phenotype of PRPS1 syndromes in females: neuropathy, hearing loss and retinopathy
Abstract
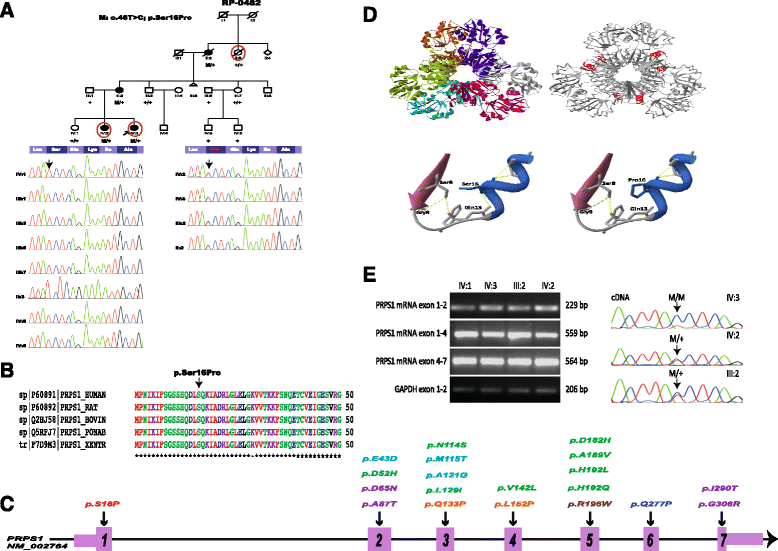
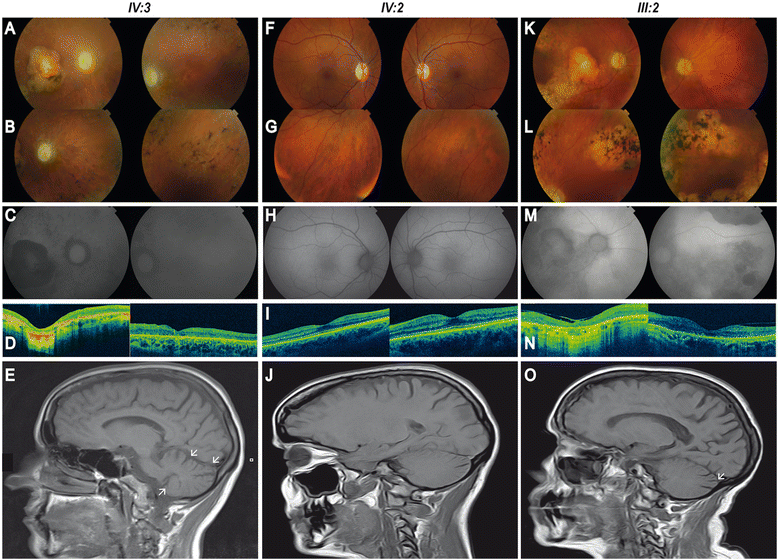
Background: Phosphoribosyl pyrophosphate synthetase (PRS) I deficiency is a rare medical condition caused by missense mutations in PRPS1 that lead to three different phenotypes: Arts Syndrome (MIM 301835), X-linked Charcot-Marie-Tooth (CMTX5, MIM 311070) or X-linked non-syndromic sensorineural deafness (DFN2, MIM 304500). All three are X-linked recessively inherited and males affected display variable degree of central and peripheral neuropathy. We applied whole exome sequencing to a three-generation family with optic atrophy followed by retinitis pigmentosa (RP) in all three cases, and ataxia, progressive peripheral neuropathy and hearing loss with variable presentation.
Methods: Whole exome sequencing was performed in two affecteds and one unaffected member of the family. Sanger sequencing was used to validate and segregate the 12 candidate mutations in the family and to confirm the absence of the novel variant in PRPS1 in 191 controls. The pathogenic role of the novel mutation in PRPS1 was assessed in silico and confirmed by enzymatic determination of PRS activity, mRNA expression and sequencing, and X-chromosome inactivation.
Results: A novel missense mutation was identified in PRPS1 in the affected females. Age of onset, presentation and severity of the phenotype are highly variable in the family: both the proband and her mother have neurological and ophthalmological symptoms, whereas the phenotype of the affected sister is milder and currently confined to the eye. Moreover, only the proband displayed a complete lack of expression of the wild type allele in leukocytes that seems to correlate with the degree of PRS deficiency and the severity of the phenotype. Interestingly, optic atrophy and RP are the only common manifestations to all three females and the only phenotype correlating with the degree of enzyme deficiency.
Conclusions: These results are in line with recent evidence of the existence of intermediate phenotypes in PRS-I deficiency syndromes and demonstrate that females can exhibit a disease phenotype as severe and complex as their male counterparts.
Figures
References
-
- Taira M, Ishijima S, Kita K, Yamada K, Iizasa T, Tatibana M. Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J Biol Chem. 1987;262(31):14867–14870. - PubMed
-
- de Brouwer AP, Williams KL, Duley JA, van Kuilenburg AB, Nabuurs SB, Egmont-Petersen M, Lugtenberg D, Zoetekouw L, Banning MJ, Roeffen M, Hamel BC, Weaving L, Ouvrier RA, Donald JA, Wevers RA, Christodoulou J, van Bokhoven H. Arts syndrome is caused by loss-of-function mutations in PRPS1. Am J Hum Genet. 2007;81(3):507–518. doi: 10.1086/520706. - DOI - PMC - PubMed
-
- Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, Jang SY, Won HH, Choi BO, Hong SH, Kim BJ, Suh YL, Ki CS, Lee SY, Kim SH, Kim JW. Mutations in PRPS1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5) Am J Hum Genet. 2007;81(3):552–558. doi: 10.1086/519529. - DOI - PMC - PubMed
-
- Liu X, Han D, Li J, Han B, Ouyang X, Cheng J, Li X, Jin Z, Wang Y, Bitner-Glindzicz M, Kong X, Xu H, Kantardzhieva A, Eavey RD, Seidman CE, Seidman JG, Du LL, Chen ZY, Dai P, Teng M, Yan D, Yuan H. Loss-of-function mutations in the PRPS1 gene cause a type of nonsyndromic X-linked sensorineural deafness, DFN2. Am J Hum Genet. 2010;86(1):65–71. doi: 10.1016/j.ajhg.2009.11.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

