Sodium sensing in the brain
- PMID: 25491503
- PMCID: PMC4325189
- DOI: 10.1007/s00424-014-1662-4
Sodium sensing in the brain
Abstract
Sodium (Na) homeostasis is crucial for life, and the Na(+) level ([Na(+)]) of body fluids is strictly maintained at a range of 135-145 mM. However, the existence of a [Na(+)] sensor in the brain has long been controversial until Nax was identified as the molecular entity of the sensor. This review provides an overview of the [Na(+)]-sensing mechanism in the brain for the regulation of salt intake by summarizing a series of our studies on Nax. Nax is a Na channel expressed in the circumventricular organs (CVOs) in the brain. Among the CVOs, the subfornical organ (SFO) is the principal site for the control of salt intake behavior, where Nax populates the cellular processes of astrocytes and ependymal cells enveloping neurons. A local expression of endothelin-3 in the SFO modulates the [Na(+)] sensitivity for Nax activation, and thereby Nax is likely to be activated in the physiological [Na(+)] range. Nax stably interacts with Na(+)/K(+)-ATPase whereby Na(+) influx via Nax is coupled with activation of Na(+)/K(+)-ATPase associated with the consumption of ATP. The consequent activation of anaerobic glucose metabolism of Nax-positive glial cells upregulates the cellular release of lactate, and this lactate functions as a gliotransmitter to activate GABAergic neurons in the SFO. The GABAergic neurons presumably regulate hypothetic neurons involved in the control of salt intake behavior. Recently, a patient with essential hypernatremia caused by autoimmunity to Nax was found. In this case, the hypernatremia was considered to be induced by the complement-mediated cell death in the CVOs, where Nax specifically populates.
Figures


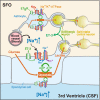
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

