The dengue virus conceals double-stranded RNA in the intracellular membrane to escape from an interferon response
- PMID: 25491663
- PMCID: PMC4261170
- DOI: 10.1038/srep07395
The dengue virus conceals double-stranded RNA in the intracellular membrane to escape from an interferon response
Abstract
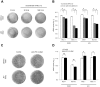
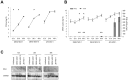
The dengue virus (DENV) circulates between humans and mosquitoes and requires no other mammals or birds for its maintenance in nature. The virus is well-adapted to humans, as reflected by high-level viraemia in patients. To investigate its high adaptability, the DENV induction of host type-I interferon (IFN) was assessed in vitro in human-derived HeLa cells and compared with that induced by the Japanese encephalitis virus (JEV), a closely related arbovirus that generally exhibits low viraemia in humans. A sustained viral spread with a poor IFN induction was observed in the DENV-infected cells, whereas the JEV infection resulted in a self-limiting and abortive infection with a high IFN induction. There was no difference between DENV and JEV double-stranded RNA (dsRNA) as IFN inducers. Instead, the dsRNA was poorly exposed in the cytosol as late as 48 h post-infection (p.i.), despite the high level of DENV replication in the infected cells. In contrast, the JEV-derived dsRNA appeared in the cytosol as early as 24 h p.i. Our results provided evidence for the first time in DENV, that concealing dsRNA in the intracellular membrane diminishes the effect of the host defence mechanism, a strategy that differs from an active suppression of IFN activity.
Figures




References
-
- Bhamarapravati N. et al. World Health Organization technical report series. Viral Haemorrhagic Fevers. Ch. 2, 13–24 (World Health Organization, Geneva, 1985). - PubMed
-
- Jessie K., Fong M. Y., Devi S., Lam S. K. & Wong K. T. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189, 1411–1418 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

