Per a 10 protease activity modulates CD40 expression on dendritic cell surface by nuclear factor-kappaB pathway
- PMID: 25492061
- PMCID: PMC4408168
- DOI: 10.1111/cei.12569
Per a 10 protease activity modulates CD40 expression on dendritic cell surface by nuclear factor-kappaB pathway
Abstract
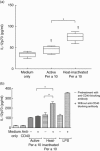
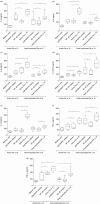
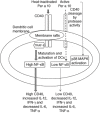
Serine protease activity of Per a 10 from Periplaneta americana modulates dendritic cell (DC) functions by a mechanism(s) that remains unclear. In the present study, Per a 10 protease activity on CD40 expression and downstream signalling was evaluated in DCs. Monocyte-derived DCs from cockroach-allergic patients were treated with proteolytically active/heat-inactivated Per a 10. Stimulation with active Per a 10 demonstrated low CD40 expression on DCs surface (P < 0·05), while enhanced soluble CD40 level in the culture supernatant (P < 0·05) compared to the heat-inactivated Per a 10, suggesting cleavage of CD40. Per a 10 activity reduced the interleukin (IL)-12 and interferon (IFN)-γ secretion by DCs (P < 0·05) compared to heat-inactivated Per a 10, indicating that low CD40 expression is associated with low levels of IL-12 secretion. Active Per a 10 stimulation caused low nuclear factor-kappa B (NF-κB) activation in DCs compared to heat-inactivated Per a 10. Inhibition of the NF-κB pathway suppressed the CD40 expression and IL-12 secretion by DCs, further indicating that NF-κB is required for CD40 up-regulation. CD40 expression activated the tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6), thereby suggesting its involvement in NF-κB activation. Protease activity of Per a 10 induced p38 mitogen-activated protein kinase (MAPK) activation that showed no significant effect on CD40 expression by DCs. However, inhibiting p38 MAPK or NF-κB suppressed the secretion of IL-12, IFN-γ, IL-6 and TNF-α by DCs. Such DCs further reduced the secretion of IL-4, IL-6, IL-12 and TNF-α by CD4(+) T cells. In conclusion, protease activity of Per a 10 reduces CD40 expression on DCs. CD40 down-regulation leads to low NF-κB levels, thereby modulating DC-mediated immune responses.
Keywords: CD40; Per a 10; allergen; dendritic cell; proteolytic activity.
© 2014 British Society for Immunology.
Figures




Similar articles
-
DC type 2 polarization depends on both the allergic status of the individual and protease activity of Per a 10.Immunobiology. 2015 Oct;220(10):1113-21. doi: 10.1016/j.imbio.2015.05.010. Epub 2015 May 11. Immunobiology. 2015. PMID: 26033313
-
Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells.Immunology. 2006 Apr;117(4):526-35. doi: 10.1111/j.1365-2567.2006.02329.x. Immunology. 2006. PMID: 16556267 Free PMC article.
-
Serine protease Per a 10 from Periplaneta americana bias dendritic cells towards type 2 by upregulating CD86 and low IL-12 secretions.Clin Exp Allergy. 2012 Mar;42(3):412-22. doi: 10.1111/j.1365-2222.2011.03937.x. Clin Exp Allergy. 2012. PMID: 22356142
-
Roles of TRAF6 in CD40 signaling.Immunol Res. 2007;39(1-3):105-14. doi: 10.1007/s12026-007-0082-3. Immunol Res. 2007. PMID: 17917059 Review.
-
The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation.Cardiovasc Res. 2010 May 1;86(2):211-8. doi: 10.1093/cvr/cvq076. Epub 2010 Mar 3. Cardiovasc Res. 2010. PMID: 20202975 Free PMC article. Review.
Cited by
-
Ingested house dust mite favors sensitization to egg white in mice independently of its proteinase activity.Front Immunol. 2025 Jan 20;15:1505003. doi: 10.3389/fimmu.2024.1505003. eCollection 2024. Front Immunol. 2025. PMID: 39902036 Free PMC article.
-
New Insights into Cockroach Allergens.Curr Allergy Asthma Rep. 2017 Apr;17(4):25. doi: 10.1007/s11882-017-0694-1. Curr Allergy Asthma Rep. 2017. PMID: 28421512 Free PMC article. Review.
-
Zinc Improves Functional Recovery by Regulating the Secretion of Granulocyte Colony Stimulating Factor From Microglia/Macrophages After Spinal Cord Injury.Front Mol Neurosci. 2019 Feb 1;12:18. doi: 10.3389/fnmol.2019.00018. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30774583 Free PMC article.
-
CXCR1 drives the pathogenesis of EAE and ARDS via boosting dendritic cells-dependent inflammation.Cell Death Dis. 2023 Sep 14;14(9):608. doi: 10.1038/s41419-023-06126-y. Cell Death Dis. 2023. PMID: 37709757 Free PMC article.
-
In Silico Comparative Exploration of Allergens of Periplaneta americana, Blattella germanica and Phoenix dactylifera for the Diagnosis of Patients Suffering from IgE-Mediated Allergic Respiratory Diseases.Molecules. 2022 Dec 9;27(24):8740. doi: 10.3390/molecules27248740. Molecules. 2022. PMID: 36557872 Free PMC article.
References
-
- Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7:363–367. - PubMed
-
- Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–316. - PubMed
-
- Ghaemmaghami AM, Robins A, Gough L, Sewell HF, Shakib F. Human T cell subset commitment determined by the intrinsic property of antigen: the pro proteolytic activity of the major mite allergen Der p 1 conditions T cells to produce more IL-4 and less IFN-γ. Eur J Immunol. 2001;31:1211–1216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

