Advances in Fecal Occult Blood Tests: the FIT revolution
- PMID: 25492500
- PMCID: PMC4366567
- DOI: 10.1007/s10620-014-3445-3
Advances in Fecal Occult Blood Tests: the FIT revolution
Abstract
There is a wide choice of fecal occult blood tests (FOBTs) for colorectal cancer screening.
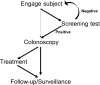
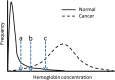
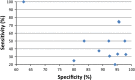
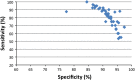
Goal: To highlight the issues applicable when choosing a FOBT, in particular which FOBT is best suited to the range of screening scenarios. Four scenarios characterize the constraints and expectations of screening programs: (1) limited colonoscopy resource with a need to constrain test positivity rate; (2) a priority for maximum colorectal neoplasia detection with little need to constrain colonoscopy workload; (3) an "adequate" endoscopy resource that allows balancing the benefits of detection with the burden of service provision; and (4) a need to maximize participation in screening. Guaiac-based FOBTs (gFOBTs) have significant deficiencies, and fecal immunochemical tests (FITs) for hemoglobin have emerged as better tests. gFOBTs are not sensitive to small bleeds, specificity can be affected by diet or drugs, participant acceptance can be low, laboratory quality control opportunities are limited, and they have a fixed hemoglobin concentration cutoff determining positivity. FITs are analytically more specific, capable of quantitation and hence provide flexibility to adjust cutoff concentration for positivity and the balance between sensitivity and specificity. FITs are clinically more sensitive for cancers and advanced adenomas, and because they are easier to use, acceptance rates are high.
Conclusions: FOBT must be chosen carefully to meet the needs of the applicable screening scenario. Quantitative FIT can be adjusted to suit Scenarios 1, 2 and 3, and for each, they are the test of choice. FITs are superior to gFOBT for Scenario 4 and gFOBT is only suitable for Scenario 1.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

