Fibronectin Matrix Assembly after Spinal Cord Injury
- PMID: 25492623
- PMCID: PMC4507358
- DOI: 10.1089/neu.2014.3703
Fibronectin Matrix Assembly after Spinal Cord Injury
Abstract
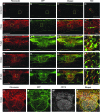
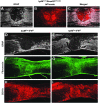
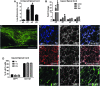
After spinal cord injury (SCI), a fibrotic scar forms at the injury site that is best characterized by the accumulation of perivascular fibroblasts and deposition of the extracellular matrix protein fibronectin. While fibronectin is a growth-permissive substrate for axons, the fibrotic scar is inhibitory to axon regeneration. The mechanism behind how fibronectin contributes to the inhibitory environment and how the fibronectin matrix is assembled in the fibrotic scar is unknown. By deleting fibronectin in myeloid cells, we demonstrate that fibroblasts are most likely the major source of fibronectin in the fibrotic scar. In addition, we demonstrate that fibronectin is initially present in a soluble form and is assembled into a matrix at 7 d post-SCI. Assembly of the fibronectin matrix may be mediated by the canonical fibronectin receptor, integrin α5β1, which is primarily expressed by activated macrophages/microglia in the fibrotic scar. Despite the pronounced cavitation after rat SCI, fibrotic scar also is observed in a rat SCI model, which is considered to be more similar to human pathology. Taken together, our study provides insight into the mechanism of fibrotic scar formation after spinal cord injury.
Keywords: fibronectin; fibrotic scar; macrophages; microglia; spinal cord injury.
Figures



References
-
- Shearer M.C. and Fawcett J.W. (2001). The astrocyte/meningeal cell interface–a barrier to successful nerve regeneration? Cell Tissue Res. 305, 267–273 - PubMed
-
- Kimura-Kuroda J., Teng X., Komuta Y., Yoshioka N., Sango K., Kawamura K., Raisman G., and Kawano H. (2010). An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol. Cell. Neurosci. 43, 177–187 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

