Tau aggregation and its interplay with amyloid-β
- PMID: 25492702
- PMCID: PMC4305093
- DOI: 10.1007/s00401-014-1371-2
Tau aggregation and its interplay with amyloid-β
Abstract
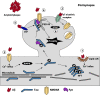
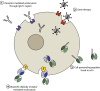
Neurofibrillary tangles and amyloid plaques constitute the hallmark brain lesions of Alzheimer's disease (AD) patients. Tangles are composed of fibrillar aggregates of the microtubule-associated protein tau, and plaques comprise fibrillar forms of a proteolytic cleavage product, amyloid-β (Aβ). Although plaques and tangles are the end-stage lesions in AD, small oligomers of Aβ and tau are now receiving increased attention as they are shown to correlate best with neurotoxicity. One key question of debate, however, is which of these pathologies appears first and hence is upstream in the pathocascade. Studies suggest that there is an intense crosstalk between the two molecules and, based on work in animal models, there is increasing evidence that Aβ, at least in part, exerts its toxicity via tau, with the Src kinase Fyn playing a crucial role in this process. In other experimental paradigms, Aβ and tau have been found to exert both separate and synergistic modes of toxicity. The challenge, however, is to integrate these different scenarios into a coherent picture. Furthermore, the ability of therapeutic interventions targeting just one of these molecules, to successfully neutralize the toxicity of the other, needs to be ascertained to improve current therapeutic strategies, such as immunotherapy, for the treatment of AD. Although this article is not intended to provide a comprehensive review of the currently pursued therapeutic strategies, we will discuss what has been achieved by immunotherapy and, in particular, how the inherent limitations of this approach can possibly be overcome by novel strategies that involve single-chain antibodies.
Figures


References
-
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci Off J Soc Neurosci. 2012;32(28):9677–9689. - PMC - PubMed
-
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

