First evidence for substrate channeling between proline catabolic enzymes: a validation of domain fusion analysis for predicting protein-protein interactions
- PMID: 25492892
- PMCID: PMC4303673
- DOI: 10.1074/jbc.M114.625483
First evidence for substrate channeling between proline catabolic enzymes: a validation of domain fusion analysis for predicting protein-protein interactions
Abstract
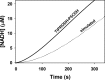
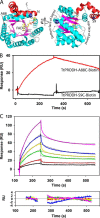
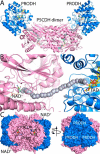
Proline dehydrogenase (PRODH) and Δ(1)-pyrroline-5-carboxylate (P5C) dehydrogenase (P5CDH) catalyze the four-electron oxidation of proline to glutamate via the intermediates P5C and l-glutamate-γ-semialdehyde (GSA). In Gram-negative bacteria, PRODH and P5CDH are fused together in the bifunctional enzyme proline utilization A (PutA) whereas in other organisms PRODH and P5CDH are expressed as separate monofunctional enzymes. Substrate channeling has previously been shown for bifunctional PutAs, but whether the monofunctional enzymes utilize an analogous channeling mechanism has not been examined. Here, we report the first evidence of substrate channeling in a PRODH-P5CDH two-enzyme pair. Kinetic data for the coupled reaction of PRODH and P5CDH from Thermus thermophilus are consistent with a substrate channeling mechanism, as the approach to steady-state formation of NADH does not fit a non-channeling two-enzyme model. Furthermore, inactive P5CDH and PRODH mutants inhibit NADH production and increase trapping of the P5C intermediate in coupled assays of wild-type PRODH-P5CDH enzyme pairs, indicating that the mutants disrupt PRODH-P5CDH channeling interactions. A dissociation constant of 3 μm was estimated for a putative PRODH-P5CDH complex by surface plasmon resonance (SPR). Interestingly, P5CDH binding to PRODH was only observed when PRODH was immobilized with the top face of its (βα)8 barrel exposed. Using the known x-ray crystal structures of PRODH and P5CDH from T. thermophilus, a model was built for a proposed PRODH-P5CDH enzyme channeling complex. The structural model predicts that the core channeling pathway of bifunctional PutA enzymes is conserved in monofunctional PRODH-P5CDH enzyme pairs.
Keywords: Amino Acid; Bacterial Metabolism; Dehydrogenase; Enzyme Kinetics; Enzyme Mechanism; Flavoprotein; Proline; Substrate Channeling.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Takagi H. (2008) Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 81, 211–223 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

